1. Background
The prevalence of thyroid nodules is very high in population reaching 19-68% of randomly selected individuals when high-resolution ultrasonography (US) is used. Although the majority of nodules are benign and the malignancy rate is relatively low (7% - 15%), it is important to differentiate malignant and benign thyroid lesions (1).
Fine needle aspiration biopsy (FNAB) of the thyroid is the gold standart diagnostic technique for determination of malignancy in thyroid nodules. It helps to avoid unnecessary surgery; however, this procedure is invasive. US is a non-invasive, cheap, easy and sensitive method for detection of thyroid nodules. Although the diagnostic value of US to differentiate between benign and malignant nodules is limited, some US features were reported to be associated with a higher risk of malignancy (2). These features are hypoechoic pattern, solid texture, presence of microcalcification, absence of peripheral halo, marginal irregularity, ratio of anterior-posterior to transvers diameter > 1, increased vascularity, and high strain index in elastosonography (3).
There are different definitions for the term “exophytic” in the literature. By the most commonly used definition, exophytic tissue is defined as a tissue growing outside towards the surface epithelium of an organ or structure in which it is originating from (4). For thyroid tissue, exophytic nodule refers to a nodule that sticks out of the normal thyroid boundary/outline. According to another definition, a nodule that makes an acute angle with the adjacent thyroid capsule could be called as exophytic nodule (5). Exophytic feature is often evaluated in many tumors. However, to our current knowledge, there is no study evaluating the exophytic feature of thyroid nodules in the literature. In this study, we aimed to determine US features, cytological findings, and the malignancy rate in exophytic thyroid nodules and compared our findings with nonexophytic ones.
2. Objectives
To evaluate US features, and cytological and histopathological findings in exophytic thyroid nodules.
3. Patients and Methods
3.1. Subjects
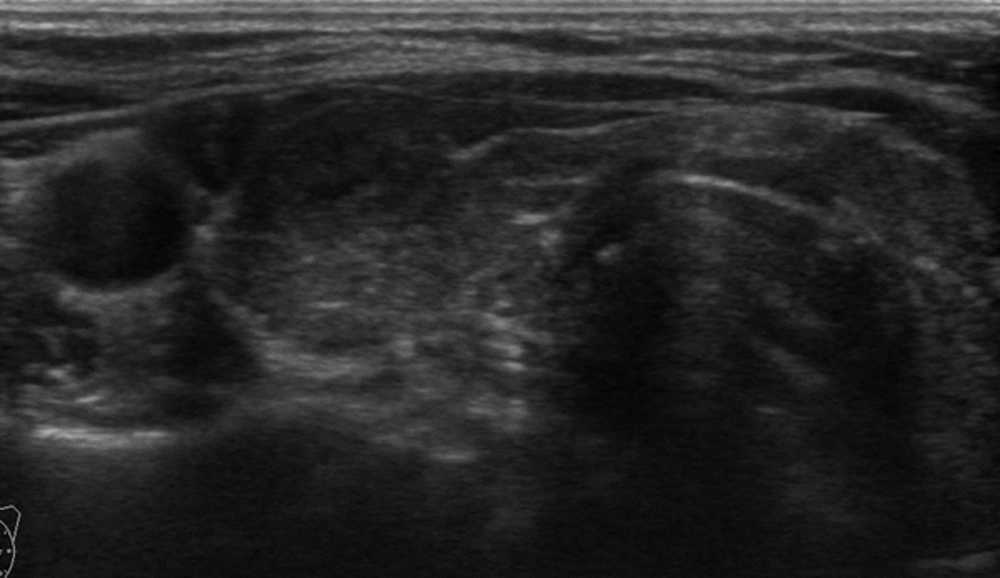
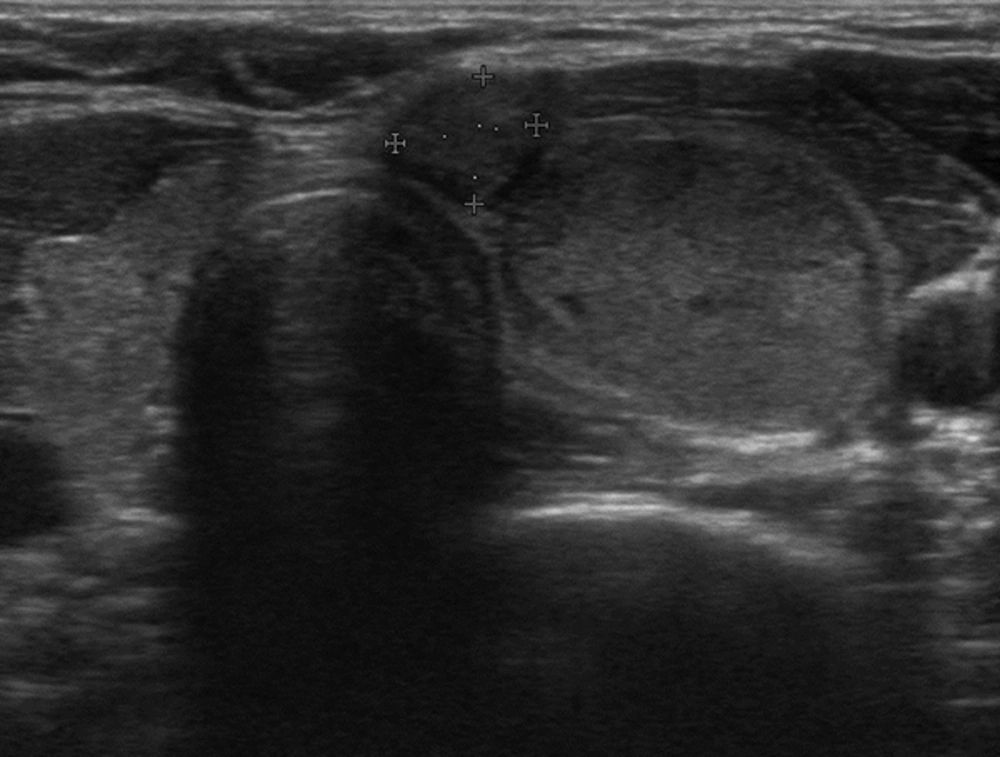
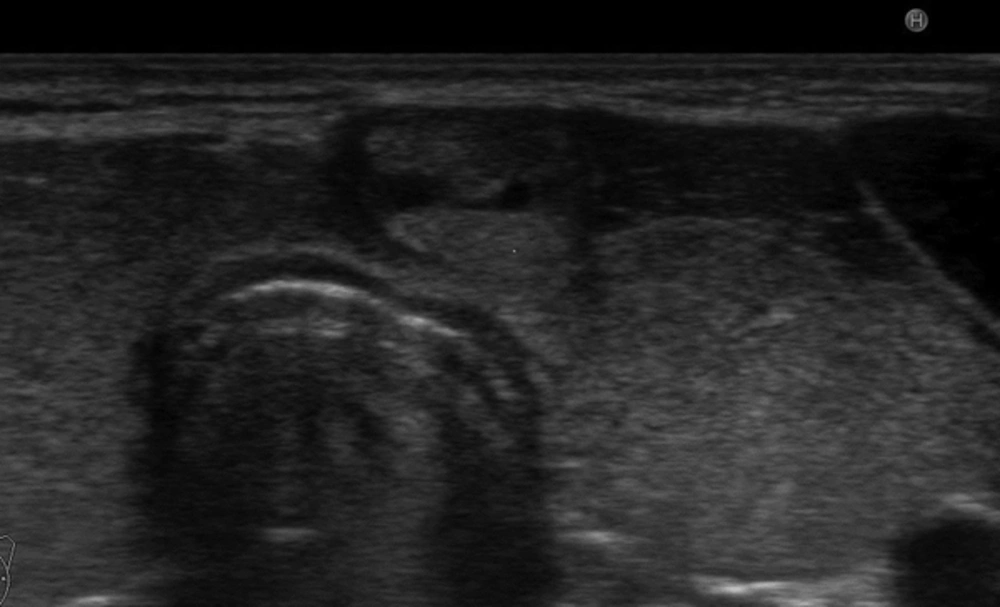
Adult patients with an exophytic thyroid nodule who underwent FNAB procedure between January 2015 and July 2015 in our clinic were recruited for this prospective study. Exophytic nodule was defined when a nodule was localized at the anterior surface of the thyroid beyond the gland borders making an acute angle with the adjacent thyroid capsule (Figure 1). Nodules larger than their originating lobe or region and nodules that overflowed outside the gland were defined as expansive nodules and excluded from the study (Figure 2). Additionally, nodules that were directly adjacent to the rigid structures such as the trachea (eg. nodules in the isthmic junction) or macrocalcific nodules/lesions which could show expansive features were excluded from the study as these nodules may falsely be considered as exophytic (Figure 3). Patients with a previous history of thyroidectomy and radiotherapy to the head and neck region were also excluded from the study. Age and sex-matched patients who had a non-exophytic thyroid nodule and underwent US guided FNAB during the same period were chosen as the control group. A total of 280 exophytic nodules in 273 patients were detected and due to exclusion criteria, data of 253 exophytic nodules in 247 patients were analyzed. Serum thyrotrophin (TSH) and thyroid hormones were measured in the last 3 months before FNAB in all patients. Antithyroid peroxidase antibody (anti-TPO) and anti-thyroglobulin antibody (anti-TGAb) were measured. US features and cytological findings of nodules were evaluated. Final histopathological diagnosis and malignancy rates of nodules were determined. Local ethical committee approved the study. All patients and control group gave written informed consent to participate in the study.
3.2. Laboratory
Chemiluminescent immunoassay was used for TSH, free triiodothyronine (fT3), free thyroxine (fT4), antithyroid peroxidase antibody (anti-TPO), and anti-thyroglobulin antibody (anti-TGAb). (Immulite 2000, Diagnostic Products Corporation, Los Angeles, CA, USA, and the UniCel DxI 800, Beckman Coulter, CA, USA). Normal ranges for TSH, fT3 and fT4 were 0.4 - 4.0 uIU/mL, 1.57 - 4.71 pg/mL, and 0.61 - 1.12 ng/dL, respectively. Anti-TPO higher than 10 U/mL and anti-Tg higher than 30 U/mL were accepted to be positive. Thyroid functional status (euthyroid/hypothyroid/hyperthyroid) and antibody positivity were compared in patients with exophytic and non-exophytic nodules.
3.3. Ultrasonography
Thyroid US was performed by an Esaote color Doppler US (Taipei, Taiwan) and compatible superficial probe (5.5 - 12.5 MHz). Parenchymal heterogenity, fibrous bands, border regularity of thyroid gland, presence of thyroiditis and parenchymal color doppler flow were evaluated. Ultrasonographically chronic thyroiditis was diagnosed when thyroid gland had a coarse, heterogeneous and hypoechoic echo pattern and/or micronodules scattered throughout the parenchyma and/or when it was permeated by fibrous echogenic layers, giving the gland a pseudolobular appearance (6, 7). Number of the nodules, localization (right or left lobe), diameters (millimeters), presence of halo, echogenity (hypoechoic/isoechoic/hyperechoic), marginal regularity (regular or irregular), presence of microcalcification and macrocalcification, and vascularization (peripheral and/or santral) were determined. US features of exophytic and non-exophytic nodules were compared. In addition, clinical and US features of histopathologically benign and malignant exophytic nodules were compared.
3.4. Fine Needle Aspiration Biopsy and Cytopathology
US-guided FNAB was carried out by an experienced clinician with a 27-gauge needle and 10 mL syringre using a Logic Pro 200 GE US machine and 7.5 MHz probe. During FNAB procedure, aspiration was performed for at least 2 - 4 times, 4 - 6 air-dried slides were prepared and sent for cytological assessment. Informed consent was taken from all patients and control group before FNAB procedure.
Bethesda classification system was used for the cytological diagnoses. The categories were nondiagnostic, benign, atypia of undetermined significance/follicular lesion of undetermined significance (AUS/FLUS), follicular neoplasm/suspicious for follicular neoplasm, (FN), suspicious for malignancy and malignant (8). In case of nondiagnostic cytology result, FNAB was repeated at least 3 months after and a nodule was considered nondiagnostic when the result was same at least for 2 times. Cytological diagnosis was compared between exophytic and non-exophytic nodules.
3.5. Histopathology
Total/near total thyroidectomy or lobectomy was performed depending on the size and/or US features and/or cytopathological result of the nodule. Postoperative histopathologic findings were classified as benign and malignant. In malignant nodules, tumor type, size and histopathological features such as multicentricity, vascular invasion, capsular invasion, extracapsular extension and lymph node metastasis were noted. Rates of malignancy were determined in exophytic and non-exophytic nodules. In addition, histopathological features of malignant exophytic and non-exophytic thyroid nodules were compared.
3.6. Statistical Analysis
All data were analyzed with SPSS Statistics for Windows, version 21.0 (Armonk, NY: IBM Corp, 2012). Descriptive analysis were presented as mean ± standard deviation for noncategorical and as number of cases and percentage for categorical variables. Noncategorical variables were compared by Student’s t test and categorical variables were compared by Chi-square test. The effect of the clinically related factors with malignancy were examined by the univariate binary logistic regression analysis. The multivariate model was constructed by using significant factors determined by the univarite analysis. The odds ratio (OR) and the 95% confidence of the OR were determined. Forward likelihood ratio was used as the variable selection method. A P value < 0.05 was accepted to indicate statistical significance.
4. Results
4.1. Clinical Features and Hormonal Status
A total of 5250 patients were evaluated by US during the study period in our clinic. Exophytic nodule was detected in 273 patients and seven patients had multiple exophytic nodules. Twenty-five patients with a history of thyroidectomy and one with a history of radiotherapy to the head and neck region were excluded from the study. Data of 247 patients with exophytic and 357 patients with non-exophytic thyroid nodules were included in the analysis. Mean age and sex were similar in the two groups (P = 0.826 and P = 0.508, respectively). There were no differences in terms of thyroid functional status, anti-TPO and anti-Tg positivity between groups (Table 1).
| Exophytic (n = 247) | Non-Exophytic (n = 357) | P Value | |
|---|---|---|---|
| Age | 48.21 ± 12.1 | 48.43 ± 12.18 | 0.826 |
| Gender | 0.508 | ||
| Female | 211 (85.4) | 297 (83.4) | |
| Male | 36 (14.6) | 59 (16.6) | |
| Function | 0.325 | ||
| Euthyroid | 195 (78.9) | 263 (73.7) | |
| Hypothyroid | 29 (11.7) | 51 (14.3) | |
| Hyperthyroid | 23 (9.3) | 43 (12.0) | |
| Anti-TPO positivity | 68 (27.5) | 113 (31.6) | 0.308 |
| Anti-TG positivity | 68 (27.5) | 115 (32.2) | 0.587 |
| Ultrasonographically chronic thyroiditis | 151 (61.1) | 147 (41.2) | < 0.001 |
| Nodule number | 4.98 ± 3.71 | 3.75 ± 2.57 | < 0.001 |
Abbreviations: anti-TG, anti-thyroglobulin antibody; anti-TPO, anti-thyroid peroxidase antibody.
aValues are expressed as mean ± SD or No (%).
4.2. Ultrasonography Features
There were 253 exophytic nodules in 247 patients and 529 non-exophytic nodules in 357 patients (Table 2). Mean nodule number and incidence of ultrasonographically detected chronic thyroiditis were significantly higher in the exophytic group compared to controls (P < 0.001 for each). Mean nodule diameter and rate of taller than wide nodules were similar in two groups (P = 0.603 and P = 0.164, respectively). Absence of peripheral halo was observed with a significantly higher rate in exophytic nodules compared to non-exophytic ones (P = 0.018). Microcalcification, marginal regularity and vascularity did not differ between groups (P = 0.085, P = 0.280 and P = 0.265, respectively). Macrocalcification was present in 30 (11.9%) of exophytic and 121 (22.9%) of non-exophytic nodules (P < 0.001). Hundred twenty four (49.4%) of exophytic and 192 (36.7%) of non-exophytic nodules were hypoechoic (P < 0.001). Solid texture was found with a lower and mixed texture was found with a higher rate in exophytic nodules compared to nonexophytic ones (P = 0.001).
| Exophytic (n = 253) | Non-Exophytic (n = 529) | P Value | |
|---|---|---|---|
| Diameter, mm, (mean ± SD) | 15.56 ± 7.00 | 15.90 ± 8.95 | 0.603 |
| Localization | 0.517 | ||
| Right | 133 (52.6) | 265 (50.9) | |
| Left | 120 (47.4) | 264 (49.1) | |
| Taller than Wider shape | 50 (19.8) | 82 (15.5) | 0.164 |
| Absence of peripheral halo | 180 (71.1) | 331 (62.6) | 0.018 |
| Microcalcification | 200 (79.1) | 432 (81.7) | 0.085 |
| Macrocalcification | 30 (11.9) | 121 (22.9) | < 0.001 |
| Echogenity | < 0.001 | ||
| Hypoechoic | 124 (49.4) | 192 (36.7) | |
| Isoechoic | 127 (50.6) | 319 (61) | |
| Hyperechoic | 0 | 12 (2.3) | |
| Texture | 0.001 | ||
| Solid | 122 (48.2) | 326 (61.6) | |
| Cystic | 13 (5.2) | 16 (3.1) | |
| Mixed | 118 (46.6) | 187 (35.3) | |
| Irregular margins | 153 (60.5) | 341 (64.5) | 0.280 |
| Vascularity | 0.265 | ||
| Peripheral | 107 (42.2) | 220 (41.6) | |
| Central | 6 (2.4) | 5 (0.9) | |
| Peripheral and central | 46 (18.2) | 54 (10.3) | |
| Absent | 94 (37.2) | 250 (47.2) | |
| Cytology | < 0.001 | ||
| Nondiagnostic | 34 (13.4) | 36 (6.8) | |
| Benign | 156 (61.7) | 468 (88.5) | |
| AUS/FLUS | 30 (11.9) | 8 (1.5) | |
| FN/suspicious of FN | 0 | 1 (0.2) | |
| Suspicios for malignancy | 16 (6.3) | 6 (1.1) | |
| Malignant | 17 (6.7) | 10 (1.9) | |
| Surgery | 73 (28.9) | 74 (14.0) | < 0.001 |
| Histopathology | N = 73 | N = 74 | 0.01 |
| Benign | 38 (52.1) | 56 (75.7) | |
| Malignant | 35 (47.9) | 18 (24.3) | |
| Histopathologically chronic thyroiditis | N = 73 | N = 74 | 0.429 |
| 26 (35.6) | 21 (28.4) |
Abbreviations: AUS/FLUS, atypia of undetermined significance/follicular lesion of undetermined significance; FN, follicular neoplasm.
aValues are expressed as No (%).
4.3. Cytological Results
There was statistically significant difference in cytology results between exophytic and non-exophytic nodules (P < 0.001) (Table 2). Benign cytology was observed in 156 (61.7%) of exophytic and 468 (88.5%) of non-exophytic nodules (P < 0.001). Nondiagnostic, AUS/FLUS and suspicious for malignancy cytologies were higher in exophytic nodules (P = 0.002, P < 0.001 and P < 0.001 respectively). Seventeen (6.7%) of exophytic and 10 (1.9%) of non-exophytic nodules were cytologically malignant (P = 0.001).
4.4. Histopathological Results
Seventy three (28.9%) exophytic nodules in 73 patients and 74 (13.9%) non-exophytic nodules in 47 controls were operated. Rate of thyroidectomy was significantly higher in the exophytic group (P < 0.001) (Table 2). Histopathologically, there was no significant difference in the presence of chronic thyroiditis between exophytic and non-exophytic nodules (P = 0.429). 35 (47.9%) of 73 exophytic nodules and 18 (24.3%) of 74 non-exophytic nodules were malignant (P < 0.01) (Table 2).
Histopathological features were available in 28 exophytic and 14 non-exophytic malignant nodules. The most common type of thyroid cancer in the two groups was papillary thyroid cancer (PTC). All malignant non-exophytic nodules were PTC, while among 28 malignant exophytic nodules, 23 were PTC, two were follicular thyroid cancer (FTC) and three were thyroid tumors of uncertain malignant potential (TT-UMP). There were no statistically significant differences between the two groups in terms of cancer type (P = 0.242). Capsular invasion was higher in exophytic group compared to non-exophytic group (53.5%, vs 14.3%, P = 0.027). There were no significant differences in terms of tumor size, vascular invasion, multicenticity, extracapsular extension, lymph node metastasis and presence of histopathological thyroiditis between two groups (Table 3).
| Exophytic (n = 28) | Non-Exophytic (n = 14) | P Value | |
|---|---|---|---|
| Tumor diameter, mm, (mean ± SD) | 10.31 ± 6.25 | 10.75 ± 5.88 | 0.810 |
| Microcarcinoma | 11 (39.3) | 7 (50.0) | 0.555 |
| Variant and type | 0.242 | ||
| PTC | 23 (82.1) | 14 (100) | |
| FTC | 2 (7.1) | 0 | |
| TT-UMP | 3 (10.8) | 0 | |
| Vascular invasion | 6 (21.5) | 0 | 0.076 |
| Capsular invasion | 15 (53.5) | 2 (14.3) | 0.027 |
| Extracapsular extension | 8 (28.6) | 2 (14.3) | 0.426 |
| Multicentricity | 12 (42.9) | 8 (57.1) | 0.382 |
| Lymph node metastasis | 1 (3.6) | 0 | 0.499 |
| Thyroiditis | 8 (28.5) | 2 (14.2) | 0.189 |
Abbreviations: FTC, Follicular thyroid cancer; PTC, Papillary thyroid cancer; TT-UMP, Thyroid tumors of uncertain malignant potential.
aValues are expressed as No (%).
4.5. Univariate and Multivariate Analysis of Ultrasonography Features
Univarite logistic regression analysis was made including all nodules with histopathological diagnosis. Exophytic appearence increased the likelihood of malignancy by 5.10 times (95% CI: 2.287 - 11.371; P < 0.001). Thyroid malignancy was significantly associated with microcalcification (OR = 2.598, 95% CI: 1.160 - 5.823), taller than wider shape (OR = 3.602, 95% CI: 1.624 - 7.999), hypoechogenicity (OR = 6.303, 95% CI: 2.506 - 15.855), solid texture (OR = 3.045, 95% CI: 1.133 - 8.183), and irregular margins (OR = 2.750, 95% CI: 1.028 - 7.359) (Table 4).
| Factors | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | % 95 CI | P Value | Odds Ratio | % 95 CI | P Value | |
| Exophytic | 5.100 | 2.287 - 11.371 | < 0.001 | 6.853 | 2.843 - 16.518 | < 0.001 |
| Taller than Wider shape | 3.602 | 1.624 - 7.999 | 0.002 | 4.629 | 1.815 - 11.809 | 0.001 |
| Absence of peripheral halo | 2.569 | 0.960 - 6.875 | 0.060 | - | - | - |
| Microcalcification | 2.598 | 1.160 - 5.823 | 0.020 | 3.759 | 1.497 - 9.439 | 0.005 |
| Echogenity (Hypo vs.Isoec) | 6.303 | 2.506 - 15.855 | < 0.001 | 5.670 | 2.156 - 14.915 | < 0.001 |
| Texture | ||||||
| Mixed. Vs. Solid | 3.045 | 1.133 - 8.183 | 0.027 | 4.886 | 1.667 - 14.316 | 0.004 |
| Cystic vs. Mixed | 0.435 | 0.049 - 3.888 | 0.456 | 0.321 | 0.031 - 3.346 | 0.342 |
| Irregular Margins | 2.750 | 1.028 - 7.359 | 0.044 | 3.445 | 1.164 - 10.193 | 0.025 |
| Vascularity | - | - | 0.311 | - | - | - |
Multivariate logistic regression analysis was performed with the significant predictors in the univariate analysis. Exopyhtic appearence (OR = 6.853, 95% CI:2.843 - 16.518), microcalcification (OR = 3.759, 95% CI: 1.497 - 9.439), taller than wider shape (OR = 4.629, 95% CI: 1.815 - 11.809), hypoechogenity (OR = 5.670, 95% CI: 2.156 - 14.915), solid texture (OR = 4.886, 95% CI: 1.667 - 14.316) and irregular margins (OR = 3.445, 95% CI: 1.164 - 10.19316) were still associated with malignancy (Table 4).
4.6. Clinical and Ultrasonography Features of Benign and Malignant Exophytic Nodules
We compared clinical and US features of histopathologically confirmed benign and malignant exophytic nodules (Table 5). There were 38 (52.1%) benign and 35 (47.9%) malignant exophytic nodule. Thyroid functional status and anti-TPO positivity were similar and anti-Tg positivity was significantly higher in malignant exophytic nodules (p = 0.071, p = 0.876 and P = 0.045, respectively). Ultrasonographically, mean nodule diameter was 19.00 ± 8.46 mm in benign and 14.67 ± 6.95 mm in malignant exophytic nodules (P = 0.020). Hypoechoic pattern was observed with a significantly higher rate in malignant nodules compared to benign ones (74.3% vs 47.4%, P = 0.019). There were no significant differences in terms of taller than wide appearence, absence of peripheral halo, texture and marginal irregularity between benign and malignant exophytic nodules.
| Benign (n = 38) | Malignant (n = 35) | P Value | |
|---|---|---|---|
| Function | 0.071 | ||
| Euthyroid | 23 (60.5) | 29 (82.9) | |
| Hypothyroid | 6 (15.8) | 1 (2.9) | |
| Hyperthyroid | 9 (23.7) | 5 (14.3) | |
| Anti-TPO positivity | 11 (28.9) | 11 (31.4) | 0.876 |
| Anti-TG positivity | 6 (15.8) | 13 (37.1) | 0.045 |
| Nodule diameter, mm, (mean ± SD) | 19.00 ± 8.46 | 14.67 ± 6.95 | 0.020 |
| Ultrasonographically chronic thyroiditis | 21 (55.3) | 18 (51.4) | 0.743 |
| Localization | 0.472 | ||
| Right | 22 (57.9) | 19 (54.3) | |
| Left | 16 (42.1) | 16 (45.7) | |
| Taller than Wider shape | 10 (26.3) | 11 (31.4) | 0.630 |
| Absence of peripheral halo | 30 (78.9) | 29 (82.9) | 0.672 |
| Microcalfication | 13 (34.2) | 8 (22.9) | 0.284 |
| Macrocalcification | 6 (15.8) | 3 (8.6) | 0.349 |
| Echogenity | 0.019 | ||
| Hypoechoic | 18 (47.4) | 26 (74.3) | |
| Isoechoic | 20 (52.6) | 9 (25.7) | |
| Texture | 0.991 | ||
| Solid | 17 (44.7) | 16 (45.7) | |
| Cystic | 19 (50.0) | 17 (48.6) | |
| Mixed | 2 (5.3) | 2 (5.7) | |
| Irregular margins | 25 (65.8) | 28 (80.0) | 0.174 |
Abbreviations: anti-TG, anti-thyroglobulin antibody; anti-TPO, anti-thyroid peroxidase antibody.
aValues are expressed as No (%).
5. Discussion
We found both cytologically and histopathologically higher rates of malignancy in exophytic nodules compared to non-exophytic ones. In addition, benign cytology was obtained only in 61.7% of exophytic nodules and nearly one third revealed indeterminate (nondiagnostic or AUS/FLUS or suspicious for malignancy) cytology. There are some US features of thyroid nodules well-known to be predictive for malignancy. These are presence of microcalcification, hypoechogenity, enhanced nodular vascularization, irregular borders, anteroposterior diameter greater than tranverse diameter and elastosonographically increased strain index (3, 9). Although these features were extensively studied previously, exophytic configuration was rarely assessed as an US feature in studies of thyroid nodules. With univariate and multivariate analysis, we showed that in addition to taller than wider shape, microcalcification, hypoechogenicity, solid texture and irregular margins, exophytic appearence was also associated with malignancy. To the best of our knowledge, this is the first study to evaluate clinical and US features and malignancy rate in exophytic thyroid nodules.
Although it is rare for thyroid, studies assessing exophytic growth pattern in non-thyroidal tumors are available in the literature. Only one study evaluated exophytic feature of thyroid nodules (5). Computerized tomography of the neck was used as the imaging method in that study and exophytic feature was observed in 6.0% of malignant and 2.2% of benign nodules. Although exophytic feature was three times more often in malignant nodules the difference did not reach statistical significance. In our study, malignancy rate was cytologically nearly 3.5 times and histopathologically nearly two times higher in exophytic nodules and the differences were statistically significant. Rate of nondiagnostic cytology was also higher in exophytic nodules. This may be related with technical difficulties due to localization and protrusion of these nodules from thyroid capsule. Additionally, AUS/FLUS and suspicious for malignancy cytologies which carry 5% - 15% and 60% - 75% risk of malignancy, respectively, were observed with significantly higher rates in exophytic group than non-exophytic group.
Hypoechogenity and absence of halo which are known to be associated with malignancy were seen with higher rates in exophytic nodules in our study. Since we showed higher rates of cytologically and histopathologically malignancy in exophytic nodules, this result was not surprising. Additionally, lower rate of macrocalcification in exophytic group which is suggested to be in favor of benign histopathology supports this finding. However, microcalcification and marginal iregularity which are associated with malignancy were similar in exophytic and non-exophytic groups. Solid consistency is more common in malignant thyroid nodules and the vast majority (82% - 91%) of thyroid cancers are solid (10-12). In a study including 360 consecutively surgically removed thyroid cancers, 88% were reported to be solid or minimally cystic (13). It is suggested that malignancy rate is higher in predominantly solid than mixed solid/cystic nodules, while cystic or spongiform ones have the lowest rate among all nodules (14). There was significant difference in texture of exophytic and non-exophytic nodules in our study and mixed texture was higher in exophytic nodules (46.6% vs 35.3%).
Prognosis and aggresiveness in exophytic configuration of different non-thyroidal tumors are controversial. Association of exophytic appearence and prognostic histopathological features has not been evaluated in thyroid malignancies previously. In our study, capsular invasion was seen with a significantly higher rate in exophytic compared to non-exophytic thyroid cancer, however there were no differences in terms of vascular invasion, extracapsular extension and lymph node metastasis. Hypothetically, exophytic nodules might have a more aggressive behaviour and exophytic appearence in US might represent capsular invasion of tumor microscopically. Gkountouvas et al. reported a case with an exophytic and ulcerated recurrence of papillary thyroid carcinoma infiltrating adjacent vital organs and skin (15).
When we compared histopathologically benign and malignant exophyic nodules, we found higher rate of anti-Tg positivity in malignant nodules, however anti-TPO positivity was similar in both. This finding was concordant with the literature which reported higher anti-TG positivity in patients with PTC compared to the general population suggesting that it is associated with increased risk for malignancy (16-18). Also, anti-TPO positivity was presented to have a protective role against thyroid cancer (19). In a recent study, TPO expression was detected to be increased in benign lesions compared to malignant ones (20). Hypoechogenity which is a feature known to be associated with malignancy was observed with higher rate in malignant than benign exophytic nodules also in our study. However, taller than wide appearence, absence of peripheral halo, microcalcification, solid texture and marginal irregularity which are believed to be suggestive for malignancy were similar in malignant and benign exophytic nodules. These features seem to lose their predictive role for malignancy in exophytic nodules. However, it is difficult to come to such a conclusion with this preliminary study.
We evaluated a large number of exophytic thyroid nodules in our study, however a limited number of patients were operated and malignancy was detected in 35 (47.9%) of them. Low number of malignant cases might be considered as a limitation. Another limitation of the present study was lack of data on prognosis, overall survival and follow-up in patients with thyroid cancer. However, our aim was to determine malignancy rate in exophytic nodules and further studies can be conducted to find out whether exophytic feature is associated with poor prognosis or not.
In conclusion, cytologically and histopathologically malignancy rates were significantly higher in exophytic nodules compared to non-exophytic ones. Although positive anti-Tg antibodies and hypoechoic apperance seem to be associated with malignancy in exophytic nodules, many other US features known to be predictive for malignancy were not higher in malignant compared to benign exophytic nodules.