1. Background
Tetralogy of Fallot (TOF) is considered as the most frequent cyanotic congenital heart disorder (1, 2). It has four characteristic components: large ventricular septal defect (VSD), right ventricular (RV) outflow tract obstruction, overriding aorta on the interventricular septum and right ventricle hypertrophy (RVH) (3, 4). The incidence of TOF is 0.3 - 0.5 in every 1,000 live births (5, 6). Tetralogy of Fallot total correction (TFTC) has shown satisfactory and significant results for 40 - 50 years (7, 8).
Despite the good long-term prognosis in post-surgical status, these patients still have shorter life expectancies compared to the normal population. An increasing number of adult patients who have had TOF repair surgery in their childhood now require regular follow up for their post-operative complications. The common post-surgical complications are pulmonic regurgitation (Figure 1), residual pulmonary stenosis, residual VSD, right ventricular outflow tract aneurysm, right ventricular dysfunction, and arrhythmia (6, 7, 9). Considering these major complications, regular follow-up after total surgical correction is necessary. Conventional imaging methods, including echocardiography and radionuclide studies are limited to assess these complications. Transthoracic echocardiography has the limitation of post-surgical scar tissue and thoracic deformities (6, 7). On the other hand, cardiac magnetic resonance (CMR) has been proposed as the gold standard imaging modality for evaluation of right ventricle function (6, 10, 11).
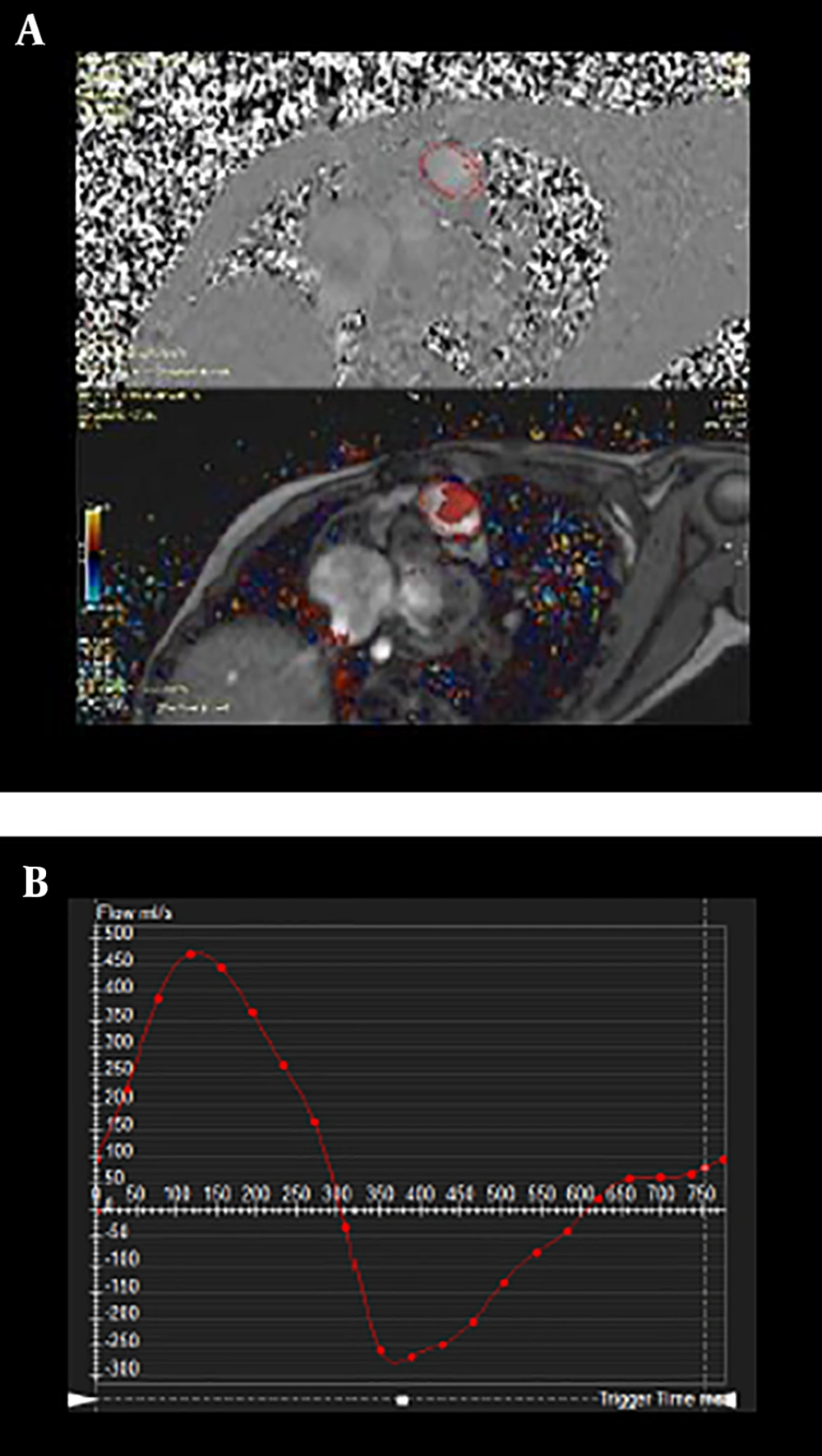
Flow measurement of a transverse slice at the level of main pulmonary artery, velocity encoded sequence on phase and magnitude (A). Flow profile of pulmonary artery; the area under the curve above the horizontal axis represents the antegrade flow and the area under the curve below the horizontal axis represents retrograde flow. The curve demonstrates severe pulmonary regurgitation (B).
Right ventricular dysfunction may occur as a serious complication in patients with complete repair of TOF with high morbidity and mortality rates (9). Different mechanisms have been proposed to contribute to global right ventricular (RV) dysfunction in patients with repaired TOF. This complication often occurs secondary to long term pulmonary valve regurgitation, which could result in right ventricular dilatation and increased risk of severe arrhythmias (6, 7). Delayed enhancement (DE) due to post-surgical scar tissue and fibrosis has been considered as an important finding in the development of right ventricular failure (12).
2. Objectives
The subject of cardiac MR in TOF patients in the Iranian society has not been explored up to now, considering the high incidence of TOF and the subsequent need for surgical treatment of these patients, this study was conducted to quantify delayed enhancement in right ventricular outflow tract (RVOT) using CMR after repairing surgery.
3. Patients and Methods
This is a descriptive analytic cross-sectional study performed on 110 consecutive symptomatic patients with repaired tetralogy of Fallot referred to Shaheed Rajaie hospital for clinical and radiological evaluation with CMR with the history of correction surgery during the past 35 years. This study evaluates clinical and CMR data from these patients. Inclusion criterion was symptomatic TOF in TFTC patients. Exclusion criteria were TOF patients with shunt surgery or inadequate clinical information for CMR. After providing full information about the methods and aims of the study, informative consent from each patient was obtained upon entry into the study. The local ethics committee confirmed the protocol of this research and it was conducted in accordance with the Helsinki declaration.
The samples were selected using the census method. All patients with sufficient inclusion criteria were enrolled in the study. The clinical and CMR data were collected from the patient registry information. The sample size was calculated by using the “one trait in the population” formula. The first type of error and the incidence of DE were assumed 5% and 70%, respectively (13).
3.1. CMR Imaging and Images Review
In this study, cardiac MR was used to estimate ventricular volumes (Figure 2) and function and also to quantify the flow vessel over the entire cardiac valves and large vessels. CMR imaging was performed on 1.5 Tesla MR system (Magnetom Avanto, Siemens Medical Systems, Germany).
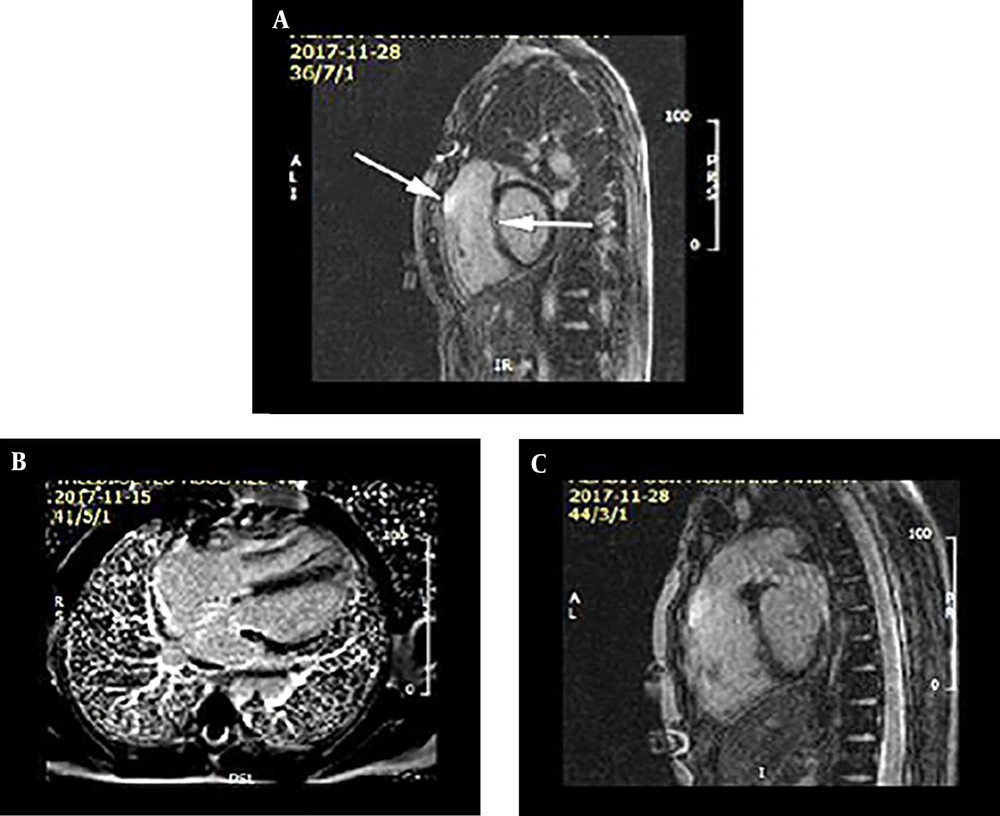
Severe right ventricular (RV) enlargement in tetralogy of Fallot total correction (TFTC). Severe increased RV volume in diastole and systole post TFTC (right ventricular end-diastolic volume [RVEDV] = 270 mL, right ventricular end systolic volume [RVESV] = 100 mL) (A). Time -volume curve of RV during cardiac cycles (B).
10 - 14 contiguous short- axis slices with a thickness of 8 mm and a gap of 25% were provided by steady-state free precession (SSFP) cine imaging covering the heart from apex to base. The software package (Argus, Siemens, Germany) was employed for measuring the ventricular end-diastolic volume (VEDV) and end-systolic volume. The data were adjusted for body size.
An additional cine imaging was used for RVOT area measurements and presented in the longitudinal axis of RVOT. The highest anterior-posterior diameter was obtained perpendicular to the long axis of RVOT at end-diastole. RVOT was considered aneurysmal if the diameter was more than 40 mm.
The regurgitation fraction was determined as regurgitation volume divided by pulmonary forward volume via pulmonary trunk velocity map. Velocity mapping was performed using a velocity encoding range of 150 cm/s. Velocity encoding was adjusted upon aliasing. The adjusted RVEF is defined as the net pulmonary forward volume divided by the right ventricular EDV (RVEDV). The net forward flow was measured by the difference between volumes of forward flow and pulmonary regurgitate.
MR imaging was carried out through single shot phase sensitive inversion-recovery technique and 0.2 millimol/kg intravenous gadoteric acid (Dotarem, Guebert, France) with 2.8 - 3 cc/sec flow injection rate 10 - 15 min following MR angiography of the pulmonary vascular tree. The following characteristics were introduced for a standard imaging: a field of view of 400 × 400 mm, a thickness of 1 mm with no gap, a [256 × 256] matrix and flip angle of 15. 20 - 30 sections were prepared through a 13 to 25 second breath hold, which was associated with the size and rate of heart in patient. An inversion time of 300 ms was set to achieve appropriate nulling of the normal myocardium through real-time imaging.
Two observers agreed on the presence or absence of delayed enhancement of the heart. They looked for DE in the free wall of the RV outflow tract, the interventricular septum and other sites of heart (Figure 3). The patients with and without DE were compared for cardiac function indices.
Phase sensitive inversion recovery sequence post gadolinium in sagittal (A, C) and four chamber axial views (B) demonstrate delayed enhancement in right ventricular outflow tract (RVOT) and ventricular septal defect (VSD) repair site in tetralogy of Fallot total correction (TFTC) represent scar in surgical site.
All statistical analyses were performed with SPSS Statistics for Windows, version 15.0 (SPSS Inc., Chicago, Ill., USA). We used frequency and ratio and also the mean and standard deviation for categorical and quantitative continuous variables, respectively. We compared proportions and means were performed using chi-2 test and t-test, respectively. Relationship between quantitative variables was determined using Pearson coefficient. P values of smaller than 0.05 were regarded as significant.
4. Results
In this study, 110 patients (65 [59.12%] male and 45 [40.94%]) female with the mean age of 21.93 ± 6.94 years were included. Of the studied patients, a seven-year old patient and a 48-year old subject were found as the youngest and the oldest patients. The mean time interval between initial surgery and CMR study was approximately 14.61 ± 6.01 years (1 - 35 years). 103 subjects (93.66%) had DE, whereas seven subjects (6.44%) did not. The data regarding frequency and location of DE are shown in Table 1.
| Location of delayed enhancement | Number of patient | Percent % |
|---|---|---|
| Variable | 81 | 73.61 |
| IVS | 1 | 0.90 |
| RVOT + right side of the septum | 4 | 3.63 |
| Right ventricular septal junction | 2 | 1.77 |
| RVOT + IVS at the site of VSD patch | 5 | 4.53 |
| RVOT + both RV septal junction | 1 | 0.87 |
| RVOT + RV apical + LV apicolateral segment | 1 | 0.89 |
| RVOT + Inferior RV septal junction | 2 | 1.81 |
| Inferior RV septal free wall junction | 2 | 1.78 |
| RVOT + anteroseptal segment | 2 | 1.73 |
| Basal septal at the side of VSD patch | 1 | 0.92 |
| RVOT + transmural apical segment | 1 | 0.97 |
| Non-delayed enhancement | 7 | 6.44 |
| Total | 110 | 100 |
Abbreviations: IVS, interventricular septum; LV, left ventricle; RV, right ventricle; RVOT, right ventricular outflow tract; VSD, ventricular septal defect
Delayed enhancement was seen in 95.47 % (62 patients) and 91.16 % (41 patients) of male and female patients, respectively. This yielded no significant gender difference (P = 0.17). The mean age for patients with and without DE were 21.92 ± 7.18 and 19.75 ± 3.68 years, respectively, which was not significant (P = 0.42).
In our study, RVOT aneurysm was seen in 99 patients (90%) and pulmonary regurgitation was found in 95 patients (86.43%). Seventy-four patients (67.37%) showed no evidence of pulmonary stenosis while the remaining 36 patients (33.72%) had pulmonary stenosis. Residual VSD was positive in 28 patients (25.56%) leaving the remaining 82 patients (74.58%) with no residual VSD. Residual VSD was usually small size in these 28 patients.
The relation between right ventricular function indices and DE is shown in Table 2. The content of this table indicates a significant relationship between DE with factors affecting right ventricular function, such as regurgitation fraction, RVEDV, right ventricular end systolic volume (RV ESV), and right ventricular ejection fraction (RV EF).
| Variable | DE positive | DE negative | P value |
|---|---|---|---|
| Regurgitation fraction | 13.91 ± 38.12 | 16.72 ± 22.36 | 0.010 |
| RV end diastolic volume | 91.88 ± 258.26 | 38.25 ± 185.51 | 0.047 |
| RV end systolic volume | 71.16 ± 162.87 | 35.96 ± 103.31 | 0.045 |
| RV ejection fraction | 7.79 ± 38.23 | 9.14 ± 44.89 | 0.048 |
| LV ejection fraction | 41.41 ± 55.25 | 4.30 ± 60.12 | 0.421 |
| LV end diastolic volume | 38.78 ± 124.93 | 20.77 ± 129.15 | 0.801 |
Abbreviations: DE, delayed enhancement; LV, left ventricle; RV, right ventricle; SD, standard deviation
aValues are expressed as mean ± SD.
The relation between DE and mean time after surgery was also investigated. The results showed that the mean duration in patients without DE was 15.68 ± 4.72 years and in patients with DE was 14.57 ± 6.29 years. Comparison of these values showed no significant difference.
5. Discussion
According to the results, DE can be found in patients with repaired TOF as well as 93.66 % of symptomatic patients after surgery showed DE. Delayed enhancement could be an indication of ventricular scarring or fibrosis in patients without ischemic heart disease. Right ventricular DE in TOF patients after surgical repair was seen in 83 % and 92% of patients in studies conducted by Oosterhof et al. and Lu et al., respectively (12, 13) which are similar to our study. A study of children with congenital heart disease (including not only TOF) carried out by Harris et al. in 2007 found DE rates of 91% after surgical repair of the anomalies. In this study, DE has been seen in 3% of children without any previous surgery (14). Babu-Narayan claimed a right ventricular DE rate at surgical site of 99% in his study, which is similar to our result of 93.66% (15). They observed left ventricular DE in 5% of the patients, which was not seen in our study. The most common sites of DE in our study were RVOT (73.61%), RVOT with other regions (21.39%) and in other areas except RVOT (5%). Incidence of DE in RVOT has been 70% in the study performed by Oosterhof et al. (12).
Most of our patients (86.4%) had pulmonary regurgitation and 36.6% of our patients had pulmonary stenosis, while in other studies, pulmonary stenosis has been reported in 10% - 15% of the subjects (10). Pulmonary stenosis has usually been seen in the proximal part of RVOT up to the distal branches, which sometimes necessitates reoperation. This difference could possibly be explained by differences in surgical techniques. These findings indicate that despite repairing surgery in patients with TOF, these post-operative abnormalities in the majority of patients ultimately would lead to RV dysfunction.
We have shown that the presence of DE has a meaningful relationship with impaired right ventricular function, while indices of left ventricular function, such as left ventricular end diastolic volume (LV EDV) and left ventricular ejection fraction (LV EF) are not influenced. It seems that left ventricular function in TOF patients with reconstructive surgery is not affected by DE. This is in par with results of previous studies. Significant relationship was seen between DE and RV dysfunction (end-systolic RV volume and RVEF) in a study conducted by Babu-Narayan et al. (15). A significant relationship between DE and RV EDV was also established in the study conducted by Lu et al. (13).
RVOT diameter and RV end-diastolic volume were increased in patients with positive DE in the study carried out by Oosterhof et al., and they both had a significant inverse relationship with RV ejection fraction (12). Wald and colleagues analyzed MRI data from 256 patients with repaired TOF and scored them based on the number of enhanced Voxels in each area. They showed that a higher DE score is associated with lower right ventricular EF (9). A significant reverse correlation between DE and RVEF was found in our study too.
Harris and colleagues evaluated DE in MRI of 73 children with congenital heart disease after surgical correction and reported significant preserved RV ejection fraction (61% ± 9%) despite the presence of DE. This difference could possibly be due to differences between pediatric and adult populations (14). In their study, DE was present in structures not directly involved in reconstructive surgery, such as ascending aorta, which could be a result of inflammation of the aortic wall. In our study, there was no DE seen in this location.
We observed no statistically significant relationship between patients’ age and gender and the incidence of DE, while Babu-Narayan et al. reported higher levels of right ventricular DE in older patients (15). Our results showed mild tricuspid regurgitation in 21 patients (19%), while Norton and colleagues found moderate to severe tricuspid insufficiency in about 10% of the patients. They concluded that tricuspid insufficiency could develop due to annular dilatation of the valve subsequent to progressive right ventricular dilation (10). Marcelo et al. found that both ventricles show abnormal high fibrosis signal after TOF repair, in contrary to our results, in their study on 30 children with a history of TOF repair, Marcelo et al. claimed that both ventricles have similar abnormal high fibrosis signal (16). This can partially be explained by considering the differences in the surgical procedures between the two studies as well as the anatomical variations in TOF patients (17), also the small sample size of their study could be another explanation for the difference seen in their results.
In conclusion, in this study we aimed to explore the subject of cardiac MR in TOF patients in the Iranian society, which has not been explored up to now and also to find the possible differences between our population and other previously studied populations. The results of our study in par with previous studies have shown that DE, which can affect the right ventricle function, is a common finding following TOF repair surgery. Therefore, CMR imaging is the modality of choice for follow up of these patients after repairing surgery.
.jpg)
![Severe right ventricular (RV) enlargement in tetralogy of Fallot total correction (TFTC). Severe increased RV volume in diastole and systole post TFTC (right ventricular end-diastolic volume [RVEDV] = 270 mL, right ventricular end systolic volume [RVESV] = 100 mL) (A). Time -volume curve of RV during cardiac cycles (B). Severe right ventricular (RV) enlargement in tetralogy of Fallot total correction (TFTC). Severe increased RV volume in diastole and systole post TFTC (right ventricular end-diastolic volume [RVEDV] = 270 mL, right ventricular end systolic volume [RVESV] = 100 mL) (A). Time -volume curve of RV during cardiac cycles (B).](https://services.brieflands.com/cdn/serve/3170b/b6830aaad04202332a40f67228a78db99487550d/iranjradiol-94150-g002-F2-preview.webp)