1. Background
As the largest sesamoid bone found in the quadriceps tendon, the patella is an uncommon location for primary and metastatic tumors of the bone (1). Primary patellar tumors account for less than 0.06% of bone tumors, and metastatic lesions to the patella are even rarer. Studies regarding patellar tumors are mostly case reports with only a few reporting a small series. Most patellar tumors are benign with giant cell tumor (33%) and chondroblastoma (16%) ranking the first and second commonest diagnosis, respectively. Osteosarcoma (6%) is the commonest primary malignant tumor in the patella, and other reported primary malignant tumors include hemangioendothelioma (3%), malignant fibrous histiocytoma (1%), and angiosarcoma (less than 1%) (1, 2). Anterior knee pain and swelling over the knee are common symptoms at presentation, most frequently associated with chondromalacia and degenerative arthritis. Moreover, resistant night pain may remind of a tumorous condition, and proper knee radiography should be performed. Radiographic identification of malignant tumors of the patella is difficult because a lot of benign lesions frequently resemble osteolytic neoplastic lesions of the patella including Paget’s disease, hyperparathyroidism, osteomyelitis, tuberculosis and gout (1-7). Patellar tumors are usually not investigated with complete radiological assessments that are very useful and necessary for sufficient staging of the tumor and for appropriate planning of treatment. Consequently, no radiological characteristics of patellar tumors in imaging have been properly recognized which may lead to misdiagnosis. Studies investigating patellofemoral pain, patellar instability and femoral trochlear dysplasia (8-11) have identified abnormalities in the patellofemoral morphological parameters including shallow trochlear groove and abnormal lateral pulley inclination. We hypothesized that abnormalities may also exist in imaging presentations in the patellofemoral morphological parameters of patients with patellar tumors.
2. Objectives
This study investigated the imaging features of patellar tumors and tumor-like lesions and the patellofemoral morphological parameters for better evaluation of these diseases.
3. Patients and Methods
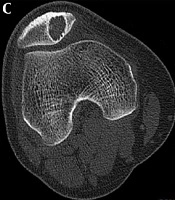
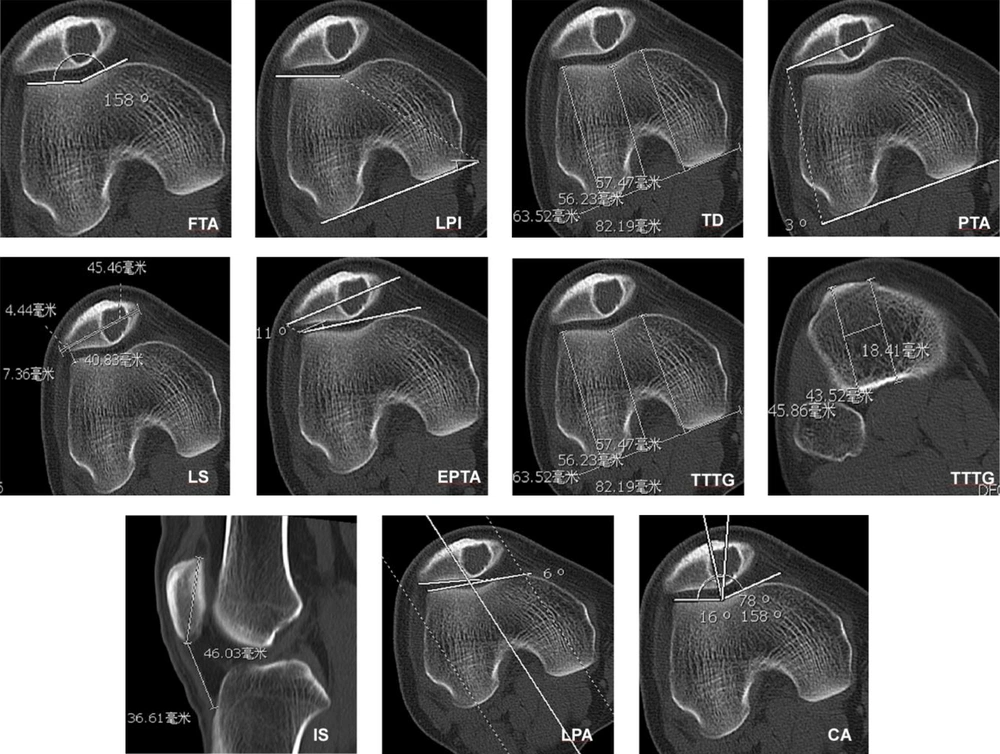
This study was approved by the ethics committee of our hospital. Informed consent was obtained from all patients for evaluation by clinical radiography. All clinical and imaging data regarding patellar tumors and tumor-like lesions confirmed by histopathology between 1976 and 2008 were retrospectively retrieved and analyzed. The inclusion criteria were patients with patellar tumors and tumor-like lesions, complete computed tomography (CT) scan data, and good imaging data for analysis. Exclusion criteria were patients without CT scan data or bad imaging data for analysis. A total of 29 patients with patellar tumors and tumor-like lesions were recognized including 20 male and nine female patients with an age range of 10 - 81 (mean, 32) years. The imaging presentations of patellar tumors and tumor-like lesions were studied. The inclusion criteria for the control group were patients without patellar diseases but with complete patellar CT scan data and good imaging data for analysis. Thus, fourteen (10 males and four females, with an age range of 10 - 81 and mean of 32 ± 19 years) patients with patellar tumors and another fifteen patients (13 males and two females with an age range of 15 - 59 and mean of 26 ± 10 years) without patellar diseases who had complete CT scan data were enrolled for measuring relevant morphologic data. Fourteen patients were set up as the tumor group, while the other 15 patients with no patellar disease were the control group. The morphological measurement on CT imaging included the femoral trochlear angle (FTA), lateral pulley inclination (LPI), trochlear depth (TD), patellar tilt angle (PTA), lateral shift (LS), external patellar tilt angle (EPTA), tibial tuberosity-trochlear groove distance (TTTG), Insall-Salvati (IS) index, lateral patellofemoral angle (LPA), and congruence angle (CA) (Figure 1) (8-12).
Measurement of morphological data of the patella with a hemangioma lesion. FTA, femoral trochlear angle; LPI, lateral pulley inclination; TD, trochlear depth; PTA, patellar tilt angle; LS, lateral shift; EPTA, external patellar tilt angle; TTTG, tibial tuberosity-trochlear groove distance; IS, Insall-Salvati; LPA, lateral patello-femoral angle; CA, congruence angle
3.1. Statistical Analysis
Statistical analysis was performed using SPSS software (version 20.0 IBM, Chicago, IL, USA). The Mann-Whitney U test was used to compare the difference between two groups. The P value < 0.05 was set as the significant level.
4. Results
4.1. Disease Classification and Distribution
Among the 29 patients with patellar tumors and tumor-like lesions, there were 20 benign lesions accounting for 69.0% of all cases and nine malignant tumors (31.0%). Benign lesions included chondroblastoma in nine patients (31.0%), giant cell tumor of the bone in four (13.8%), chondroma in one (3.4%), hemangioma in one (3.4%), parosteal lipoma in one (3.4%), bone cyst in two (6.8%), aneurysmal bone cyst in one (3.4%), and fibrous dysplasia in one (3.4%). Malignant tumors were bone metastasis in four patients (13.8%), osteosarcoma in three (10.3%) including common osteosarcoma in two and well-differentiated osteosarcoma in the remaining one, chondrosarcoma in one (3.4%) and hemangiopericytoma in one (3.4%).
Nine patients had chondroblastoma in an age range of 10 - 43 (mean, 21) years including eight males, with six patients (66.7% or 6/9) in the age range of 15 - 20 years and eight (88.9% or 8/9) in the age range of 10 - 30 years. Giant cell tumor of the bone was in four patients with an age range of 21 - 41 (mean, 30) years and a male-to-female ratio of 1:1. The metastatic lesions of the patella occurred in an age range of 18 - 81 (mean, 46) years with a male-to-female ratio of 3:1. The patellar osteosarcoma had an age range of 22 - 58 years (36) with a male-to-female ratio of 2:1. Other lesions were less frequent.
4.2. Morphological Measurements
No significant (P > 0.05) difference existed in the mean age and sex distribution between the tumor and control group. In the tumor group, the trochlear groove was abnormally shallow and flat (Figures 2-8). The FTA was significantly (P < 0.001) greater in the tumor than in the control group (159.19º ± 9.42º vs. 135.00º ± 5.50º), whereas the LPI (14.69º ± 8.91º vs. 20.73º ± 3.51º, P = 0.01) and TD (3.10 ± 1.79 vs. 6.67 ± 1.26 mm, P < 0.001) were significantly smaller in the tumor group than in the control group. No significant difference existed in the other measurement indexes (P > 0.05) (Table 1).
| Groups | FTA (º) | LPI (º) | TD (mm) | PTA (º) | LS | TT-TG (mm) | IS index | LPA (º) | CA (º) | EPTA (º) |
|---|---|---|---|---|---|---|---|---|---|---|
| Case | 159.19 ± 9.42 | 14.69 ± 8.91 | 3.104 ± 1.79 | 9.51 ± 5.9 | 0.14 ± 0.08 | 14.51 ± 6.05 | 1.09 ± 0.35 | 8.55 ± 4.81 | 13.06 ± 6.59 | 9.92 ± 6.24 |
| Control | 135.00 ± 5.50 | 20.73 ± 3.51 | 6.67 ± 1.26 | 7.93 ± 3.88 | 0.09 ± 0.04 | 18.96 ± 9.24 | 1.12 ± 0.19 | 11.06 ± 5.14 | 11.53 ± 5.9 | 10.06 ± 4.84 |
| Z | -4.59 | -2.60 | -3.99 | -0.67 | -1.82 | -1.81 | -0.29 | -1.81 | -0.41 | -0.14 |
| P | < 0.001 | 0.01 | < 0.001 | 0.50 | 0.07 | 0.27 | 0.77 | 0.07 | 0.06 | 0.89 |
Abbreviations: CA, congruence angle; EPTA, external patellar tilt angle; FTA, femoral trochlear angle; IS, Insall-Salvati; LPA, lateral patello-femoral angle; LPI, lateral pulley inclination; LS, lateral shift; PTA, patellar tilt angle; TD, trochlear depth; TT-TG, tibial tuberosity-trochlear groove distance
aNote: The rank sum test with the Z value for statistical comparison
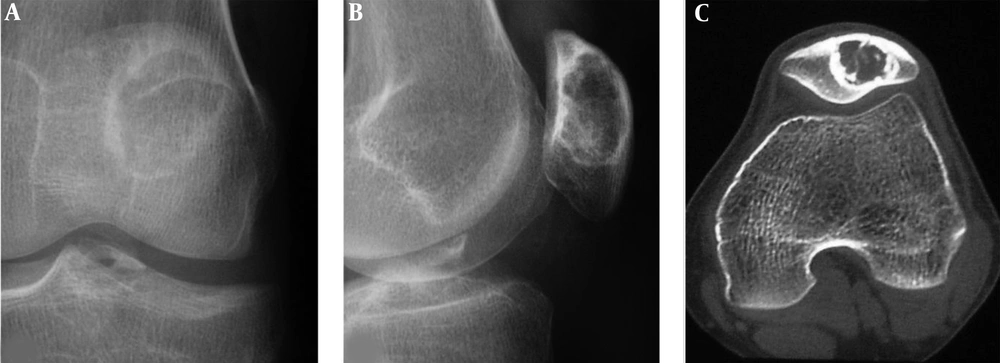
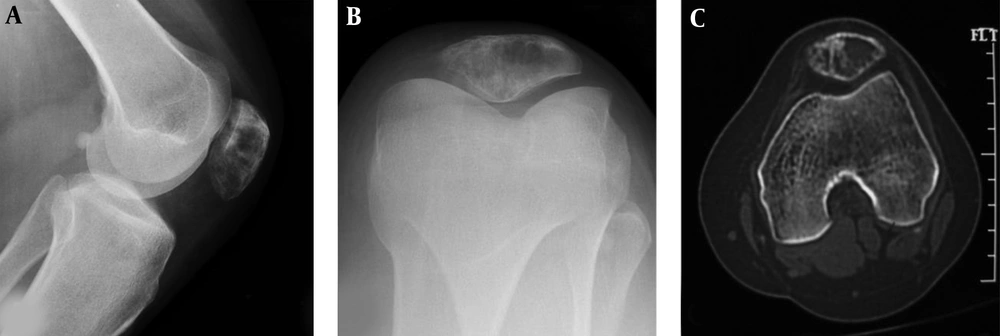
A patellar chondroblastoma is demonstrated in a 19-year-old boy who had knee pain for 2 years. Plain radiographs (A and B) and computed tomography (C) shows a lucent lesion in the left patella with sclerotic rims, inhomogeneous density and irregular calcification inside the lesion. The trochlear groove is shallow (C).
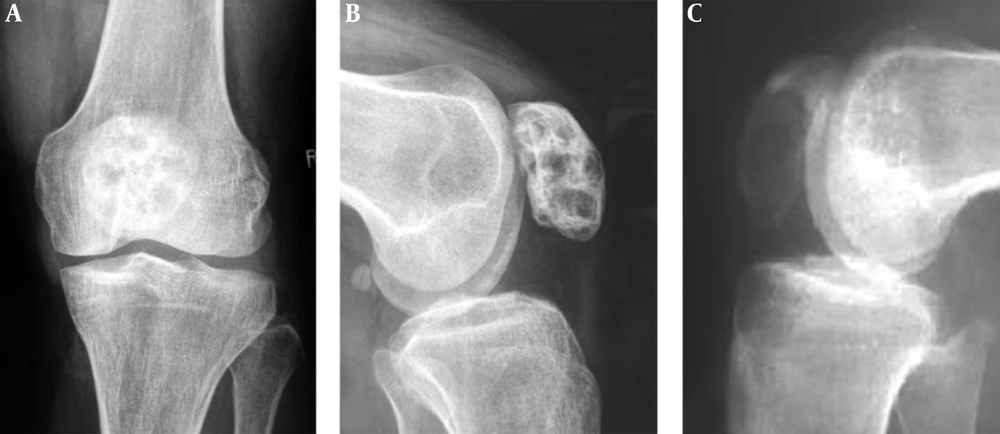
Patellar chondroblastoma and giant cell tumor are shown. A and B, A 19-year-old boy with left knee pain for two months. A, A round bone destruction area is shown with slight sclerotic rims and homogeneous density inside the lesion. B. The short tau inversion recovery (STIR) sequence of magnetic resonance imaging revealed the lesion to be long T2 weighted image signal with inhomogeneous signal, clear rims and effusion inside the joint capsule. C and D, A giant cell tumor is shown in the irregular patella with a lytic destruction area and swelling joint capsule (C). D, Computed tomography shows an eccentric bond destruction area with expansive thinned cortex. The trochlear groove is shallow, the medial and lateral pulley inclination (LPI) is small, and the lateral pulley joint surface is longer.
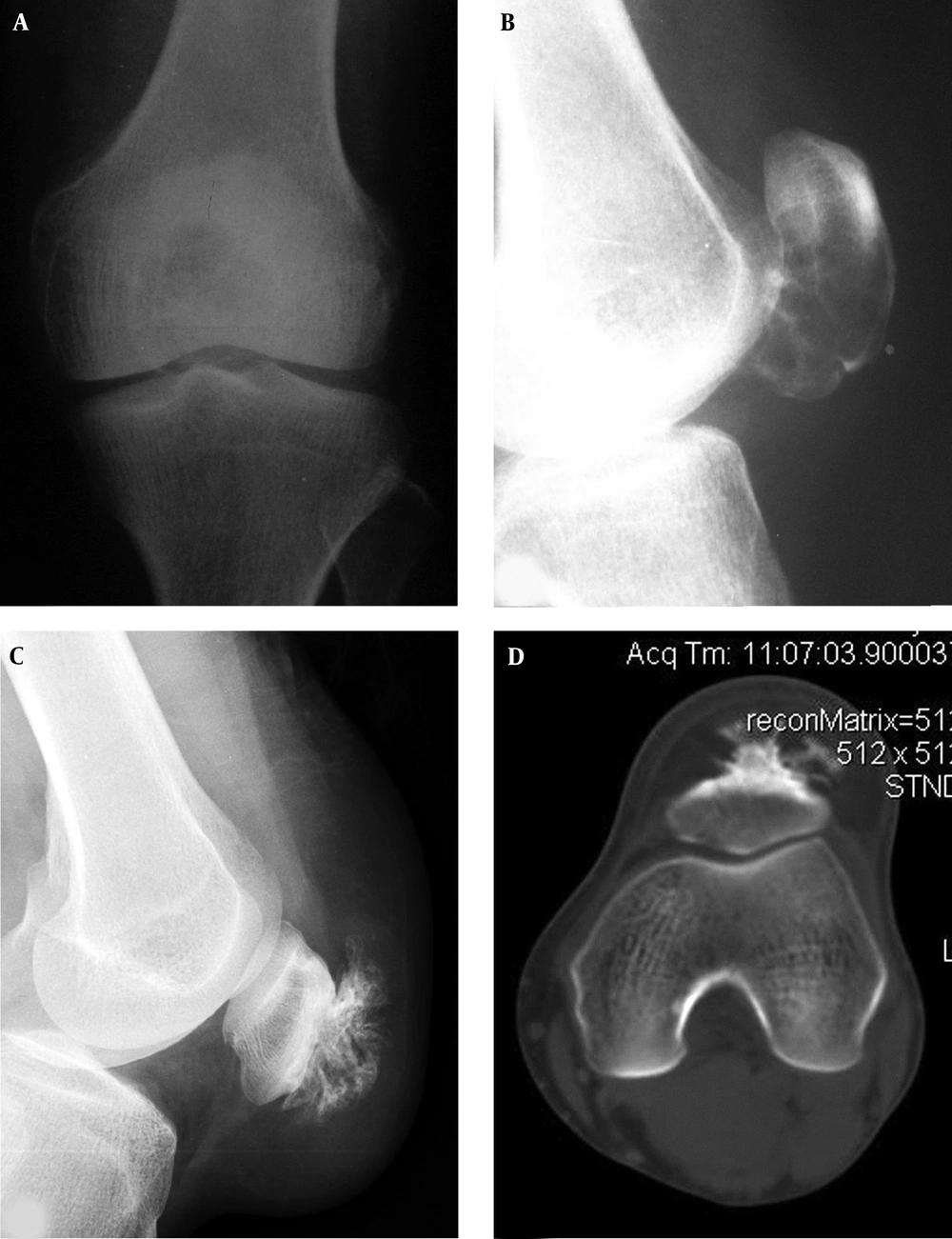
A well-differentiated osteosarcoma is shown in the patella. A and B, Radiographs demonstrate decreased density in the lower extremity of the patella with interrupted cortex. C, Computed tomography reveals expansive lytic destruction with thinned cortex and interrupted cortex in the interior bottom similar to a benign lesion. The trochlear groove is relatively shallow with a smaller LPI (lateral pulley inclination).
Patellar fibrous dysplasia and bone cyst are demonstrated. A and B, Plain radiographs reveal inhomogeneous destruction like a beehive in the patella with coarse septations and high density inside the lesion. C, A patellar bone cyst demonstrates it as lytic destruction with relative homogeneous density inside the lesion.
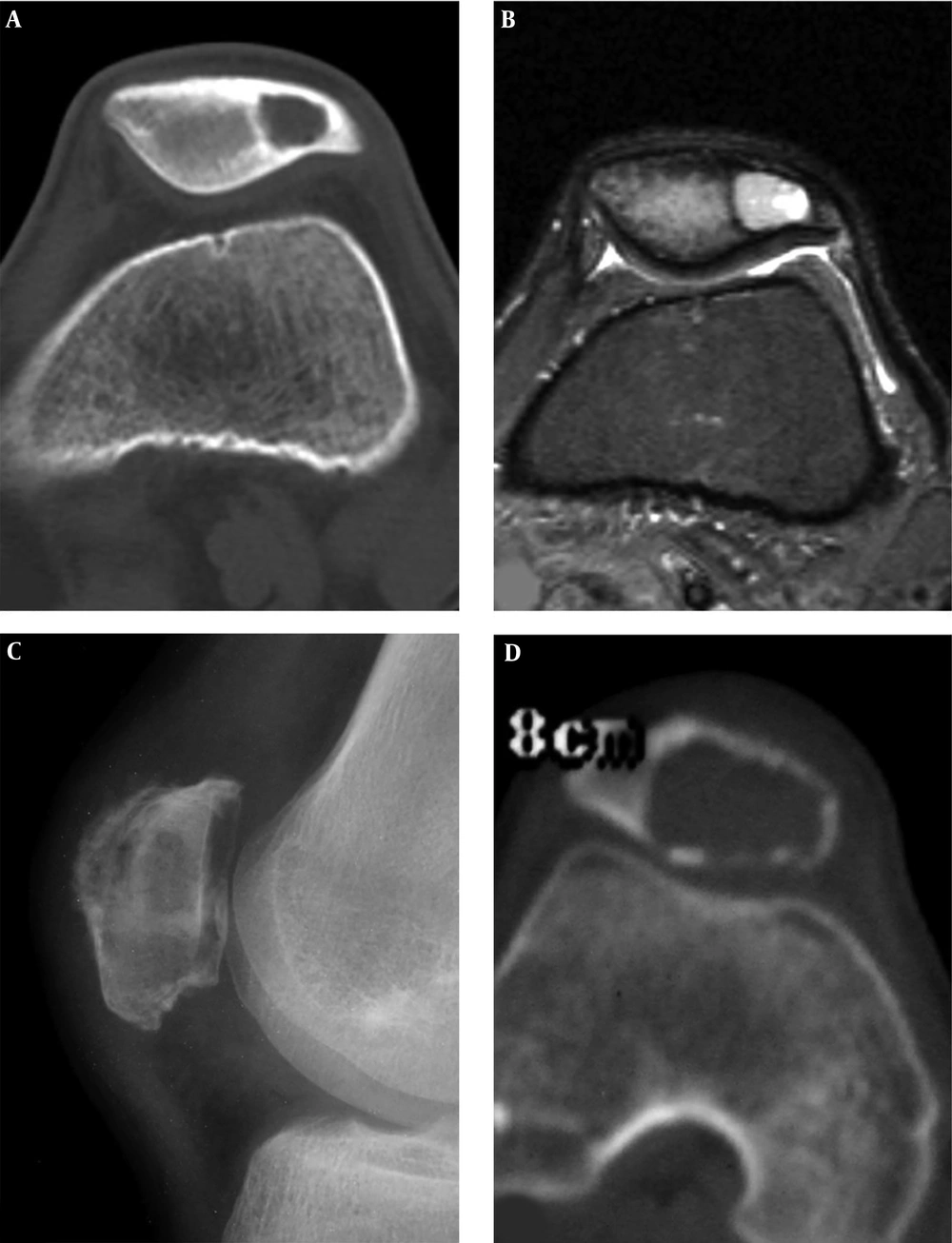
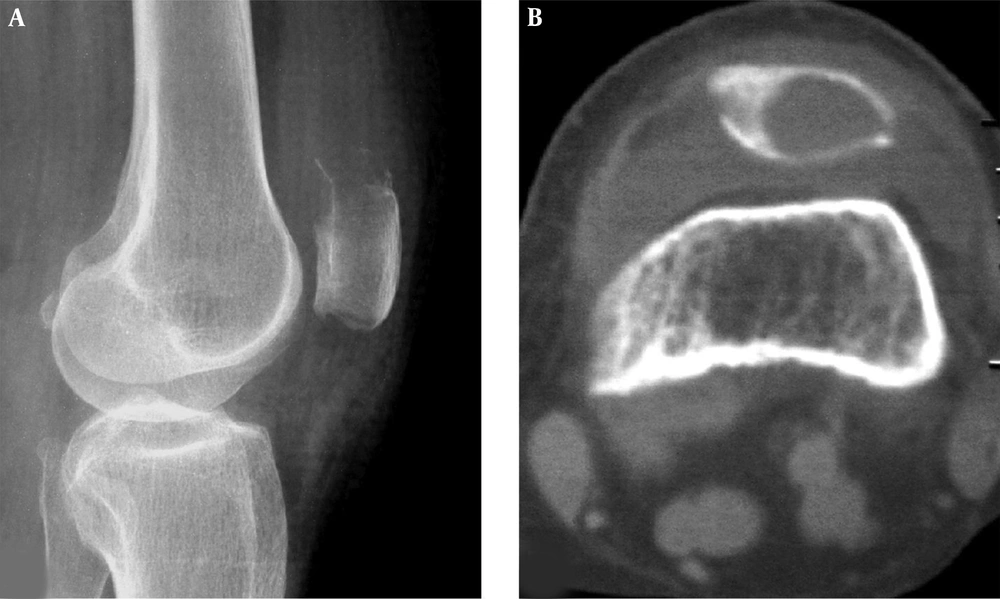
Patellar aneurysmal bone cyst and parosteal lipoma are shown. A and B, The patellar aneurysmal bone cyst demonstrates an expansive lytic destruction area in the lower half of the patella with fine and thin septations inside the lesion. C and D, The parosteal lipoma is shown as soft tissue in front of the patella with fatty density and branches of bone protrusions connected to the patella front part. The trochlear groove is relatively shallow with smaller lateral pulley inclination (LPI) (D).
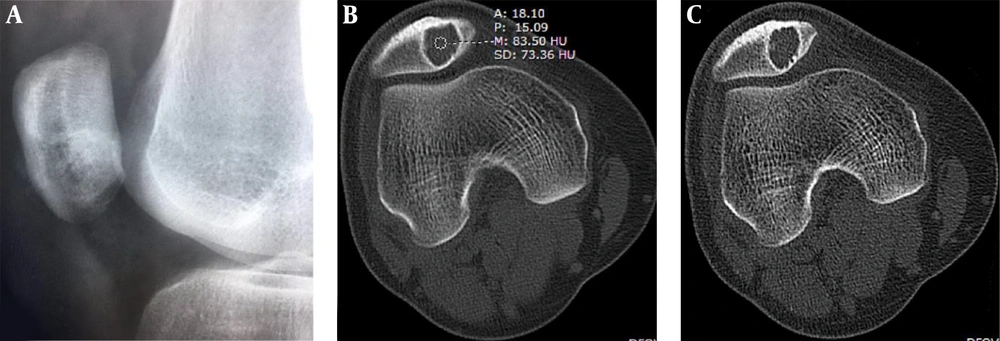
Patellar hemangioma. Plain radiograph (A) and CT scan (B and C) demonstrate an eccentric lesion with well-defined, sclerotic margins. The CT value inside the lesion is relatively high (B) with interior calcification (C). Bone crests are formed at the edge (C). The trochlear groove is shallow and flat with a smaller LPI (lateral pulley inclination).
4.3. Imaging Presentations of Diseases
The bone lesion of chondroblastoma was relatively small with a size range of 1 - 3 cm and slight expansion in imaging (Figures 2 and 3). The lesion was located in the center of the patella in four patients but eccentrically located in the other five patients. Round or oval osteolytic lesions were typical findings with a sclerotic rim of reactive bone and occasional irregular calcification within the lesion. CT scanning was sensitive to displaying fine calcification.
For giant cell tumor of the bone, the osteolytic lesion ranged from 2 to 6 cm and was expansive with thinned expanded cortex, clear and non-sclerotic margins and no calcification inside the lesion (Figure 3). In plain radiographs, the lesion margins were unclear and like an osteolytic lesion of malignancy because of overlapped tissues.
For adenocarcinoma metastatic cases, the lesion was lytic at the upper extremities of the patella with unclear margins (Figure 4), and a soft tissue mass appeared in one patient. The osteosarcoma lesion presented in imaging as mixed destruction of bone with unclear margins, eroded cortex and a soft tissue mass in two cases. In the remaining case with well-differentiated osteosarcoma, the lesion was lytic in imaging with unclear margins like a benign lesion (Figure 5). In the one case with fibrous dysplasia, the lytic lesion had unspecific imaging with multiple septa and inhomogeneous density inside the lesion, giving a beehive appearance with an expanded cortex, coarse septations inside the lesions and sclerotic rims (Figure 6). The aneurysmal bone cyst lesion was expansively lytic at the lower extremity of the patella with homogeneous density and small thin septations in the lesion but no sclerotic rims (Figure 7). The parosteal lipoma presented as a fatty tumor in front of the patella with the base connected to the patella by ossified branches (Figure 7). The hemangioma lesion was small with sclerotic rims, high CT values ranging from 60 hounsfield units (HU) to 90 HU and interior calcification (Figure 8). Chondrosarcoma had calcification inside the lesion in some cases. No specific imaging features were found in the other tumors.
5. Discussion
In this study, the trochlear groove was abnormally shallow and flat in the tumor group, the FTA was significantly (P < 0.001) greater in the tumor than in the control group (159.19º ± 9.42º vs. 135.00º ± 5.50º), whereas the LPI (14.69º ± 8.91º vs. 20.73º ± 3.51º, P = 0.01) and TD (3.10 ± 1.79 vs. 6.67 ± 1.26 mm, P < 0.001) were significantly smaller in the tumor group than in the control group. No significant (P > 0.05) difference existed in the other parameters regarding patellar shift and dislocation (P > 0.05), which may indicate no dislocation or shift of patella in patellar tumors and tumor-like lesions. Significantly, different FTA, LPI and TD in the tumor group may indicate femoral trochlear dysplasia that is a geometric abnormality of the depth and morphology of the trochlear groove primarily at its cranial part. Femoral trochlear dysplasia may cause patellar instability which in turn leads to injury to the patellofemoral joint and anterior knee pain, consequently playing a role in the development of patellar tumors and tumor-like lesions (8, 10).
Patellar tumors were rare, and among 6322 unreported cases with tumors and tumor-like lesions in the skeletal system that had been confirmed by histopathology in our hospital in a span of 45 years, only 29 cases were patellar tumors accounting for only 0.45% of the cases. Benign patellar tumors were more frequent than malignant ones in this study (69.2% vs. 30.8%), similar to what has been reported in the literature (73% of benign vs. 27% of malignant tumors) (1). Studies conducted by Moser et al. (13) and Singh et al. (14) also confirmed that patellar tumors are mostly benign. The most common benign patellar tumor has been reported to be giant cell bone tumor (14) or chondroblastoma (13), but in our study, the most common type was chondroblastoma followed by giant cell tumor. Among 279 cases of benign patellar tumors reported by Mercuri et al. (1), the prevalence of benign tumors was in the following order: giant cell bone tumor in 126 cases, chondroblastomas in 61, chondromas in 19, primary aneurysmal bone cysts in 15, bone cysts in 14, osteoid osteomas in 10, osteoblastomas and osteochondromas in nine, hemangiomas in eight, ganglions in four, lipomas in two, nonossifying fibroma in one, and osteoma in one. For malignant tumors, the most common type was metastases followed by osteosarcoma, similar to what has been reported in the literature (1, 13-16). Mercuri et al. reported 105 cases of patellar malignant tumors with metastases in 45 cases, osteosarcoma in 25, lymphoma in 14, hemangioendothelioma in 11, malignant fibrous histiocytoma in six, plasmacytoma in two, and angiosarcoma in two (1).
The onset age range of benign and malignant tumors of the patella overlapped in this study, with relatively younger patients of chondroblastoma between 10 and 20 years of age, which was nearly ten years younger than that for giant cell bone tumor. The age range for giant cell tumor of the bone was 21 - 41 years with a mean of 30 years. Malignant patellar tumors are very rare with the frequent bone metastatic lesion occurring in older patients with a mean age of 42 years in our study. The mean age of osteosarcoma was different in the patella compared to the long bone in teenagers, with the mean age in patella greater at a mean age of 36 years. Except for giant cell bone tumor, other tumors had a greater prevalence in male than in female patients, especially for chondroblastoma.
The main symptom at presentation is unilateral anterior knee pain, similar in nature to pain caused by degenerative osteoarthritis or chondromalacia (2). Benign tumors usually have a long history of over one year. The disease history is very important in the early diagnosis of benign lesions especially the nature and duration of pain, with rest pain at night in particular being one crucial clue. Malignant patellar tumors have no apparent swelling, pain or bone destruction in early periods but progress very rapidly mostly in older patients whose diagnosis is frequently delayed because the symptoms are often mistaken as degenerative osteoarthritis. Significant delay in diagnosis usually occurs due to the non-specific complaint and rarity of patellar tumors, with the mean duration of 17 (4 - 36) months between symptom onset and definitive diagnosis (2). Subtle radiological alterations can readily be neglected at initial assessment.
Chondroblastoma accounts for 1% - 3% of benign primary tumors of the bone with 4% - 6% occurring in the patella (13, 14, 17). Most chondroblastomas arise in the epiphysis of an immature long bone in the second decade of life. In our series with nine cases, the man age was 21 years at presentation (range, 10 - 43 years). The radiographic appearance of chondroblastoma was relatively small with slight expansion, a sclerotic rim of reactive bone and irregular calcification within the lesion in 30% - 50% of the cases. These imaging features were significantly important for correct diagnosis.
About 5% - 9% of all primary bone tumors are giant cell bone tumors, and of all these tumors in the skeletal system, less than 1% happens in the patella typically in the third decade of life (18, 19). The imaging characteristics in the patella are not different from other bone locations but are usually greater in size compared with chondroblastomas. Giant cell bone tumors are cystic lytic bone destruction with expansive thinned cortex, no calcification inside the lesion, clear rims but no apparent sclerosis of the rim. Septations may be seen inside the lesion. Giant cell bone tumors are locally aggressive with 1% - 2% pulmonary metastases being reported (14). In our case series, none of these tumors had metastases.
Patellar metastatic tumors are less frequent than primary ones, and the rarity of these metastases have been attributed to the relatively distal localization and fairly poor blood supply of this sesamoid bone. In metastasis, the most common primary tumor is lung carcinoma probably through direct arterial dissemination similar to the phalanges (2, 20). The patellar metastatic lesion is mostly a lytic destruction with ill-defined margins, lacking periosteal reaction and sclerotic rims and accompanied with a soft tissue mass. Nonetheless, the imaging appearance may also be mixed with osteogenic and osteolytic appearance. Osteosarcomas in the patella have no specific imaging features and can present as osteosclerotic and osteolytic destruction with different extents, erosion of the cortex, and a soft tissue mass.
Patellar tumors or tumor-like lesions must be differentiated in radiological imaging from other bone tumors. Osteoblastomas are cystic expansive destruction with sclerotic rims and calcification and may be difficult to be differentiated from giant cell tumors in cases with no calcifications. Osteochondromas are bony masses mostly located at the distal end of the patella with a wide base to connect with the patella. Other diseases in the patella are extremely rare. Bone cysts may be like an expansive cyst with possible pathological fractures and fluid-fluid level in the lesion. Aneurysmal bone cysts are beehive-like lytic destruction with small fine septations inside the lesion but no sclerotic rims, and these signs are quite different from those of fibrous dysplasia which is of a beehive-like lytic lesion with coarse septations and sclerotic rims. The patellar hemangioma lesion was similar to chondroblastoma in imaging; however, patellar hemangioma occurs in relatively older patients with interior calcification, high CT values inside the lesion and bone crests at the lesion edge. Other diseases in the patella do not have particular imaging characteristics, and when plain radiographs are normal, computed tomographic scans are very useful since they can precisely demonstrate the nidus and location of the lesion.
In conclusion, the patellar tumors are mainly benign and rarely malignant. Comprehensive understanding of the distribution, incidence and imaging features of patellar tumors and patellofemoral morphological parameters could improve the diagnostic accuracy.