1. Context
Phantom limb pain (PLP) is a distressing phenomenon that is commonly encountered by individuals who have undergone amputation. The condition is distinguished by the experience of electric-like pain and discomforting sensations, such as painful cramps, shooting, and burning sensations in the non-existent area of the body (1-3). Furthermore, residual limb pain, also known as stump pain, is a form of pain that is perceived to emanate from the remaining portion of an amputated limb (1).
In Western societies, cancer or traumas are frequent etiologies for amputation, and only a minority of patients present with congenital anomalies or septic infections. The major cases of limb amputations are executed due to vascular ailments or diabetes mellitus (4). Amputations are primarily linked to traumatic injury on a global scale. Such injuries can be attributed to accidental events, including workplace incidents and road accidents (5). The prevalence of PLP among individuals who have undergone limb amputation is reported to range from 50% to 80%, according to published rates. However, it has been noted that a minority of these individuals, approximately 5 - 10%, suffer from severe pain for a long time (6, 7). The management of PLP poses a significant challenge, as existing pharmacological and non-pharmacological interventions have demonstrated limited to moderate efficacy (8).
Non-invasive brain stimulation (NIBS) techniques, including transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS), have been employed to alleviate pain in diverse chronic intractable pain syndromes (9, 10). These techniques stimulate the activation of the cerebral cortex by means of magnetic fields or low-intensity direct currents. This process induces a shift in the polarity of the neuronal membrane, resulting in the emergence of spontaneous neuronal activity and the modulation of cortical excitability (11). Given notable advancements in neuroscientific instruments for comprehending disease pathophysiology, our review focused on the existing hypotheses regarding the operational mechanism of NIBS, the potential of such methods as a neuroscientific instrument for exploring PLP, and the substantiated effectiveness of these techniques in PLP management. We reviewed all studies applying tDCS and TMS in patients with PLP to offer a comprehensive perspective on past, current, and emerging research in this field.
2. Evidence Acquisition
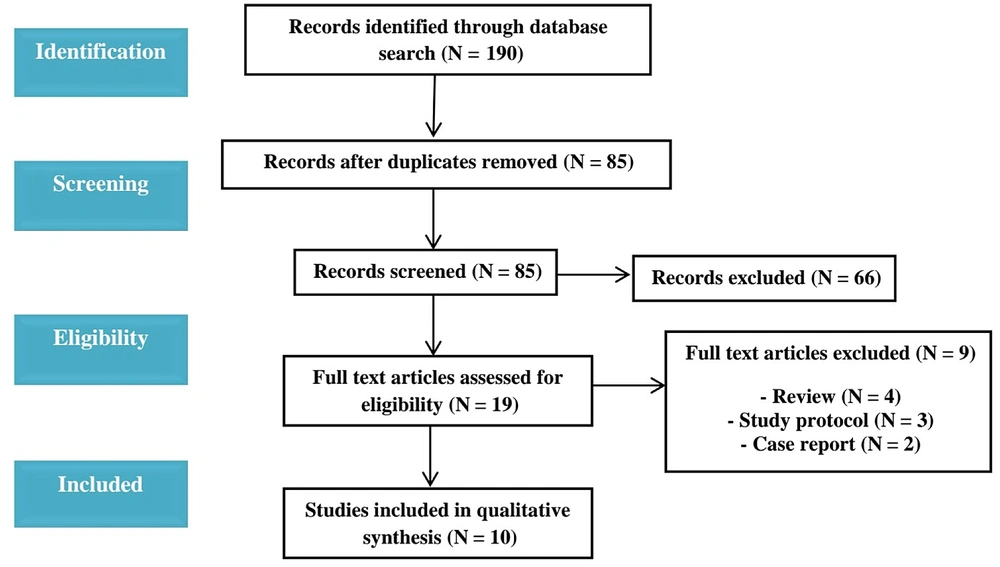
PubMed, Google Scholar, and Web of Science were searched for studies from Jan 1, 2003 to 2021. Various keywords and their combinations were used regarding PLP, neuromodulation, transcranial direct current stimulation, transcranial magnetic stimulation, and pain management. Non-English research and studies focusing on peripheral nervous system stimulation were excluded based on the criteria. Review articles were also excluded. Hence, all English literature encompassing human clinical trials in adult populations, spanning phantom limb pain and central nervous system stimulation, was included from Jan 2003 to Dec 2021. One hundred ninety studies were initially gathered, and after removing duplicates, 85 studies remained. A critical examination of the title and abstracts was then conducted for these retained studies. Following the initial screening, 10 studies were identified and subsequently read in full text (Figure 1). Table 1 summarizes the review of ten studies arranged chronologically. Among these, seven utilized tDCS, while the remaining three employed rTMS. The primarily coded variables included reference, number of patients and controls, intervention, treatment location, and duration, the pain relief assessment scale, and study results and conclusions.
| Reference | Design | Population; Age; Sex, Cause of Amputation | Number of Participants | Intervention Protocol | Stimulation Location | Number of Sessions | Outcome Parameters | Results |
|---|---|---|---|---|---|---|---|---|
| Segal et al. (2021) (12) | RCT | Age: 58.1, range 21 – 82; Sex: M = 23, F = 7; Cause: Traumatic = 11, ischemic = 2, cancer = 1 | 30 | Anodic tDCS, 1.5 mA, 0.04 mA/cm2, 20 min. MT: During tDCS, same sessions and duration: (1) MT; (2) MT and sham tDCS; (3) MT and tDCS | Anode over contralateral M1 to the affected limb. Cathode over the homolateral SOA. | 10 sessions | Brief Pain Inventory, Sensory subscale of the Short Form McGill Pain Questionnaire, Pain intensity. | Active tDCS resulted in a reduction of intensity in PLP; sham tDCS didn't reduce the PLP intensity |
| Gunduz et al. (2021) (13) | RCT | Age: 44.29; sex: M = 74, F = 38; cause: No data | 112 | Anodic tDCS, 2 mA, 0.057 mA/cm2, 20 min | Anode over contralateral M1 to the affected limb. | 10 sessions | Beck Anxiety Inventory, BDI, VAS for PLP, residual limb pain; phantom limb sensation | Active tDCS, as opposed to sham, resulted in the reduction of PLP intensity. No discernible change in depression or anxiety. No modifications in PLS were observed in either of the groups. |
| Kikkert et al. (2019) (14) | Crossover RCT | Age: 47 ± 3; sex: M = 24, F = 8; cause: Trauma = 13, tumor = 1, vascular = 1 | 32 | Anodic tDCS, sham tDCS, 1 mA, 0.028 mA/cm2, 20 min | Anode over contralateral M1 to the affected limb. Cathode over the homolateral SOA | 1 anodic, 1 sham tDCS randomized sessions | PLP frequency, PLP intensity (short pain questionnaire) | Anodic tDCS over the primary motor cortex (M1) of the missing hand resulted in significant and sustained relief of PLP in both the short and longer term. The observed effects persisted for a minimum of one week. |
| Bocci et al. (2019) (15) | Crossover RCT | Age: 21 (24 - 58); sex: M = 6, F = 8; cause: Traumatic = 11, ischemic = 2, cancer = 1 | 14 | Anodic tDCS, sham tDCS. 2.0 mA, 0.057 mA/cm2, 20 min | Anode: Bilaterally over the cerebellar area. Cathode: Right shoulder | 5 real and sham tDCS randomized sessions | Paroxysmal pain, stump pain, PLS, phantom movements, VAS PLP intensity | tDCS was found to decrease paroxysmal pain significantly, non-painful PLS, and movements in individuals experiencing these symptoms. |
| Bolognini et al. (2015) (16) | Crossover RCT | Age: Range 22 – 76; sex: M = 3, F = 5; cause: Ischemic = 5, traumatic = 2, cancer = 1 | 8 | Anodic tDCS, sham tDCS, 1.5 mA, 0.043 mA/cm2, 15 min | Anode over contralateral M1 to the affected limb. Cathode over the homolateral SOA | 5 anodic, 5 sham tDCS randomized sessions | frequency of PLP paroxysms, BDI, VAS for PLP intensity, phantom limb movement, non-painful PLS, | Active tDCS, as opposed to sham, resulted in a persistent reduction in both the intensity and frequency of PLP, and the effect was observed to persist for one week following the cessation of treatment. The BDI didn’t demonstrate any significant improvements in PLS. |
| Bolognini et al. (2013)A (17) | Crossover RCT | Age:59, range 22 – 77; sex: M = 4, F = 4; Cause: Ischemic = 6, traumatic = 2 | 8 | Anodic, sham tDCS. 2.0 mA, 0.057 mA/cm2, 15 min | Anode over contralateral M1 to the affected limb. Cathode over the homolateral SOA | 1 anodic, 1 sham tDCS randomized sessions | VAS for telescoping, VAS for nonpainful phantom limb, VAS for stump pain, VAS for PLP | Anodal tDCS to the primary motor cortex (M1) resulted in a specific and temporary reduction of PLP. |
| Bolognini et al. (2013)B (17) | Crossover RCT | Age:57.6, range 22 – 77; sex: M = 4, F = 3; cause: Ischemic = 5, Traumatic = 2 | 7 | Anodic, cathodic, sham tDCS. 2.0 mA, 0.057 mA/cm2, 15 min | Active electrode over the contralateral parietal area to the affected limb. Reference electrode over the homolateral SOA | 1 anodic, 1 cathodic, 1 sham tDCS randomized session. | VAS for nonpainful phantom limb, VAS for telescoping, VAS for stump pain, VAS for PLP | Cathodic tDCS on the PPC resulted in a temporary reduction of PLS. The observed alterations in sensory perception returned to pre-stimulation levels within 90 minutes. |
| Ahmed et al. (2011) (18) | RCT | Age: 52.2; sex: M = 19, F = 8; cause: Traumatic = 6, Ischemic = 8, Diabetic = 13 | 27 | Experimental group: Real rTMS once per 10 min (200 pulses at 20 Hz) and 80% of motor threshold, eight coil Control group: Sham rTMS with the same parameters | over the identified the motor cortical area corresponding to the stimulated stump muscle of painful side | 5 consecutive days | LANSS Pain Scale | rTMS administered at a high frequency over the motor cortex for five consecutive days has been shown to offer enduring pain relief in individuals experiencing phantom pain. This therapeutic effect may be attributed to an elevation in central nervous system endorphins. |
| Malavera et al. (2016) (19) | RCT | Age: 67.8 ± 8.25; sex: M = 50, F = 4; cause: Traumatic (landmine victims) (n = 54) | 54 | Experimental group: real rTMS of M1 contralateral to the amputated leg was given in a series of 20 trains of 6 s of duration (54 s inter-train, intensity 90% of motor threshold applied through a figure of eight coil) at a stimulation rate of 10 Hz (1200 pulses), 20 min/day, during 10 days Control group: Same stimulation parameters (location and duration) with a sham coil | Experimental group: M1 contralateral to the amputated leg Control group: Same stimulation parameters (location and duration) with a sham coil | 2 weeks | VAS | The application of 10 Hz rTMS on the contralateral primary motor cortex for a duration of 2 weeks in individuals with traumatic amputation and phantom limb pain (PLP) results in a noteworthy reduction in pain that is clinically significant for up to 15 days post-treatment. |
Abbreviations: BDI, becks depression inventory; F, female; LL, lower limb; M, male; MT, mirror therapy; PLP, phantom limb pain; PLS, phantom limb sensations; RCT, randomized controlled trial; SOA, supra-orbital area; UL, upper limb; VAS, Visual Analogue Scale.
2.1. Phantom limb pain and Central Nervous System Neuromodulation Techniques
The onset of PLP typically is shortly after a surgical procedure, although it may be delayed in certain patients. Painful phantom sensations are typically sporadic and endure for a brief period, ranging from seconds to minutes, but may persist for several hours or even indefinitely (3, 20, 21). A significant percentage of patients experience persistent sensations for an extended period (22). From a clinical perspective, PLP frequently manifests in the distal regions of the absent limb, such as the hand, fingers, foot, or toes. This may be attributed to the greater representation of distal body parts in the somatosensory cortex in comparison to the proximal limb (23, 24). Although PLP is typically categorized as neuropathic, patients frequently describe their pain as a nociceptive pain sensation, such as shooting, crushing, or squeezing, or the sensation like a car driving over their foot (25).
2.1.1. Classification
It is imperative to note that not all post-amputation painful sensations are attributed to phantom pain. For its proper management, it is crucial to differentiate and evaluate these sensations through an interdisciplinary approach. Phantom limb pain is undoubtedly one of the three potential presentations that may occur after amputation (26):
1. Phantom limb pain is a distressing sensation that's experienced in the absence of a limb that has been amputated.
2. Stump pain refers to the experience of localized pain in the residual limb following amputation.
3. Phantom sensations refer to any sensory experience other than pain that is perceived in the absence of a limb.
2.1.2. Underlying Mechanisms
Previously, PLP was generally considered a psychiatric disorder. However, with the extensive research conducted over the past few decades, the hypothesis has shifted towards recognizing alterations at various levels of the neural axis, particularly the cortex (27). In recent years, numerous potential mechanisms have been identified for the development and perpetuation of PLP. However, the persistence of PLP is probably a complex, multifaceted process influenced by bodily, psychological, and social factors. However, there appear to be widespread underlying factors in the form of inevitable nerve damage, resulting in corresponding peripheral and central alterations within the nervous system (24, 28, 29). During the process of amputation, the peripheral nerves are incised, leading to significant tissue and neuronal damage and neuroma development at the end of the injured nerve (30). This leads to significant damage to both tissue and neurons, resulting in the disturbance of the typical afferent nerve input pattern to the spinal cord. Subsequently, a procedure known as deafferentation occurs, whereby the proximal segment of the severed nerve generates neuromas (27). These neuromas exhibit an augmented buildup of molecules that amplify the manifestation of sodium channels, leading to hyperexcitability and spontaneous discharges (31). It is believed that the atypical peripheral activity may serve as a plausible origin of stump pain, which encompasses PLP (27). This hypothesis is further supported by research demonstrating the effectiveness of sodium channel-blocking drugs in alleviating PLP (32, 33).
In this phenomenon, evidence has demonstrated increased activity within the spinal pronociceptive excitatory structures, accompanied by an increase in the activity of glutamate and the N-methyl-D-aspartate (NMDA) receptor system facilitated by neurotransmitters such as tachykinins, neurokinins, and substance P at the dorsal horn of the spinal cord (34-36). The aforementioned process induces an alteration in the pattern of nociceptive-related neurons firing in the central nervous system. A decrease in the local inter-segmental inhibitory mechanisms at the spinal cord level may occur, leading to spinal dis-inhibition and the transmission of nociceptive inputs to the supra-spinal centers. The absence of afferent input and alterations at the spinal cord level is proposed as the underlying mechanism of PLP (35, 37, 38).
In recent years, cortical reorganization has emerged as a frequently cited explanation for the etiology of PLP. The cortical reorganization, including its process and extent, has been the subject of investigation in animal and human models after deafferentation and amputation. The degree of cortical reorganization has been observed to have a direct correlation with the level of pain experienced and the magnitude of the differentiated area. Several imaging studies have established a correlation between a larger extent of somatosensory cortex involvement and more intense pain (39-41).
2.2. Treatment Options
The available treatments comprise pharmacotherapy, adjuvant therapy, and surgical intervention. There is a range of medication options available, such as tricyclic antidepressants (TCAs), NSAIDs, and opioids. Among these options, TCAs are frequently utilized as a primary treatment. Several therapeutic approaches based on varying principles are suggested for the effective management of PLP. Although there are currently no established treatment protocols, effective pain management and rehabilitation interventions often demand a multi-disciplinary approach (42).
2.2.1. Pharmacological Approaches
Pharmacological intervention has been established as the primary approach for addressing PLP in amputees, utilizing various agents, including opioids, amitriptyline, gabapentin, and local anesthetics (43, 44).
Opioids can bind to both peripheral and central opioid receptors, thereby providing analgesic effects without compromising consciousness, the sense of touch, and proprioception. The efficacy of opioids, such as oxycodone, morphine, and methadone, in managing neuropathic pain, including PLP, has been supported by randomized controlled trials. Comparative studies have demonstrated the advantages of opioids over tricyclic antidepressants and gabapentin. However, opioid use is linked with a higher incidence of adverse effects (45, 46).
TCAs are frequently prescribed medications for the treatment of various neuropathic pains, including PLP. The analgesic effects of tricyclic antidepressants are primarily attributed to their ability to inhibit the uptake of serotonin and norepinephrine, antagonizing NMDA receptors, and blocking sodium channels (47). A recent study demonstrated that the management of PLP was exceptionally and consistently successful, with an average dosage of 55 mg of amitriptyline. However, in some studies, tricyclic antidepressants were ineffective in managing pain (45, 48). A limited case series has exhibited the efficacy of mirtazapine, an alpha 2 receptor antagonist that presents fewer adverse effects compared to TCAs in managing PLP (49).
The efficacy of gabapentin in controlling PLP has yielded mixed results, with some studies demonstrating positive outcomes and others indicating no efficacy (50-52). Carbamazepine has been documented to alleviate the acute stabbing and piercing pain commonly associated with PLP. Despite the availability of pharmacologic agents, many continue to experience refractory pain and require invasive or noninvasive treatment options (53).
2.2.2. Non-invasive Neuromodulation
There exist non-invasive treatment modalities available for PLP, including sensory motor training, mirror visual therapy, and non-invasive neuromodulation. Non-invasive neuromodulation comprises interventions such as transcranial direct current stimulation (tDCS) and transcranial magnetic stimulation (TMS). These techniques involve the stimulation of the cerebral cortex through the application of magnetic fields or weak direct currents. This stimulation alters the polarity of the neuronal membrane, resulting in spontaneous neuronal activity and modulation of cortical excitability. The excitability of the cortex can be modulated through the application of tDCS, which can either increase or decrease cortical activity depending on the polarity and scalp-site localization of the stimulation. Similarly, the frequency and intensity of pulse repetitions for TMS can also influence cortical excitability (11).
2.2.2.1. Transcranial Direct Current Stimulation
Neurons are cells capable of electrical excitation, and their proper functioning depends on the production of action potentials. Action potentials are generated upon the attainment of a specific potential threshold during the depolarisation of the resting membrane. The determination of the potential of the neuronal membrane is contingent upon afferent activity through both electrical and chemical synapses, as well as the presence of extrasynaptic substances that activate specific ion channels and receptors (54, 55). The function of tDCS is to directly modify the resting potentials of neurons, thereby changing their level of excitability. This alteration affects the likelihood of afferent activity of a particular magnitude, resulting in the generation of an action potential. In the event of depolarisation of a neuronal membrane by a direct current, the threshold for inducing an action potential is lowered, thereby reducing the need for afferent activity. Conversely, hyperpolarisation of the membrane results in a reduction of neuronal excitability and a subsequent decrease in spontaneous activity (56-58). It is imperative to acknowledge that this mode of operation is fundamentally distinct from supra-threshold stimulation, which triggers an action potential at a compromised neuronal membrane, as develops in TMS (59).
The application of tDCS can alternate cortical excitability and activity. Specifically, when the anode is positioned over the primary motor cortex (M1), tDCS amplifies both spontaneous activity and excitability, thereby serving as a model region. On the other hand, if the cathode is positioned above M1, there is a decrease in both spontaneous activity and excitability (60, 61). It is noteworthy that the modification in resting membrane potentials caused by tDCS at conventional intensity (1 - 2 mA) is comparatively minimal. Transcranial direct current stimulation is suggested to modify neuronal membrane potential by approximately 0.2 to 0.5 Mv (62, 63). At first glance, the seemingly negligible alteration between the resting membrane potential of approximately -70mV and the threshold for action potential initiation of approximately -50mV may be observed. It has been postulated that tDCS may be effective despite the modest impact it has on membrane potential at the specific neuron level (64). This may be attributed to amplification due to alterations in action potential generation within larger neuronal networks, modulation of action potential timing, or both. Both of these mechanisms are activated during neuronal network stimulation, resulting in comparable alterations in voltage (65, 66).
Bolognini et al. conducted one of the initial investigations on the management of PLP utilizing tDCS. In their study, the impact of a solitary session of anodal versus sham stimulation over M1 was evaluated on eight participants. The research findings indicate that anodal tDCS has a brief analgesic impact on PLP, lasting up to 90 minutes post-stimulation (17). The administration of this therapeutic intervention elicits a subthreshold alteration in the resting membrane potentials, which tends to shift towards either depolarisation or hyperpolarisation. This phenomenon is believed to regulate neuroplasticity. Numerous theories have been put forth regarding the neuroplastic mechanisms of tDCS that have been reported. These theories encompass both connectional and local effects (67). Transcranial direct current stimulation has recently emerged as a nonpharmacological treatment for chronic pain, including PLP. This method of stimulation is safe and noninvasive and has been proven effective with minimal contraindications (10, 67).
2.2.2.2. Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) is a non-invasive technique utilized for the electrical stimulation of nerves, encompassing the spinal roots, cerebral cortex, as well as peripheral and cranial nerves (68-71). Transcranial magnetic stimulation can be administered in the form of singular pulses of stimulation, pairs of stimuli with varying intervals to either the different or same regions of the brain, or as repetitive trains of stimuli at different frequencies. A solitary stimulus can depolarize neurons and elicit quantifiable outcomes. The application of repetitive TMS in the form of trains of stimuli has been shown to induce alterations in the excitability of the cerebral cortex not only at the site of stimulation but also in remote regions connected through functional anatomical pathways. Transcranial magnetic stimulation has the potential to offer innovative perspectives into the pathophysiology of the neural circuitry that underlies neurological and psychiatric disorders. Furthermore, it can be a clinically valuable diagnostic and prognostic test and have therapeutic applications in a variety of diseases. The TMS potentials mentioned above are demonstrated by extensive research, but further investigation is required to definitively ascertain the precise function of TMS in clinical neurology (59, 72-74). The process involves the application of a brief, high-intensity current (measuring several thousand amps) through a copper wire coil. This, in turn, generates a magnetic field of up to approximately 2T, which persists for approximately 100ms. The magnetic field pulses emanating from a stimulating coil applied to the scalp can traverse the skull bone without any attenuation, thereby generating an electric field into the brain. The magnitude of the induced current is adequate to elicit action potentials and stimulate cerebral networks securely and painlessly (73, 74). Transcranial magnetic stimulation is a valuable tool for investigating cortical plasticity and reorganization patterns, as well as the underlying mechanisms driving these neuroplastic changes (75).
Several research groups have utilized rTMS as a non-invasive means of predicting the clinical response following epidural implants for chronic neuronal stimulation. This approach is particularly relevant in cases of chronic pain, tinnitus, and, potentially, movement disorders. However, a prudent approach, according to the rules of evidence-based medicine, would be welcome for the described therapeutic applications, and rTMS is being tested for diseases with a fairly heterogeneous physiologic basis, and it seems unlikely that rTMS will be good for all (76-78). When examining the analgesic effectiveness of rTMS in the context of chronic pain, it is imperative to take into account various factors. These factors include but are not limited to the frequency of stimulation, the specific site of stimulation, the duration of stimulation, and the delay between the stimulation and the observed clinical effects (76). The variation of excitability resulting from rTMS is dependent on the frequency of stimulation. In general, high-frequency stimulation with a frequency of 5 Hz or greater is known to induce an excitatory response. In comparison, low-frequency stimulation with a frequency of 1 Hz or less is observed to decrease cortical network excitability in various conditions. It is important to acknowledge that the outcomes of rTMS can be subject to variability, contingent upon the particular targets of stimulation and the pre-existing level of activation within the circuits being targeted (79). Transcranial magnetic stimulation techniques have the potential to provide a dependable means of characterizing significant neurophysiological and pathophysiological aspects of brain involvement in individuals experiencing PLP and phantom sensations (PS) (80). The various paradigms of TMS have yielded valuable insights into distinct neurotransmitter systems, thereby augmenting our comprehension of the underlying pathophysiology of these conditions. In general, the studies utilizing TMS indicate a correlation between PLP and neuroplastic alterations (81, 82). In a clinical trial conducted by Lefaucheur et al., it was determined that the application of rTMS over M1 at a frequency of 10 Hz was significantly effective in alleviating neuropathic pain. However, no such observation was made at a frequency of 0.5 Hz (83). According to another group of researchers, the use of rTMS was found to be more productive in mitigating pain when administered at a frequency of 20 Hz, as opposed to 1 Hz (84).
3. Discussion
This study aimed to review CNS neuromodulation techniques in managing pain among individuals with PLP. There is substantial evidence regarding the plastic changes that occur in the human motor system after amputation. Phantom limb pain has been linked to a maladaptive restructuring of the sensorimotor cortex, which is marked by a disturbance in intra-cortical inhibitory mechanisms and the existence of an imbalance between the levels of inhibitory and excitatory amino acids, specifically gamma-aminobutyric acid (GABA) and glutamate, which increase the excitability of corticospinal neurons (85-87). In 2003, Töpper et al. conducted a clinical trial on the effects of rTMS on pain syndromes. The study focused on two patients who suffered from chronic arm pain due to unilateral lower cervical root avulsion. Additionally, four healthy participants were subjected to induced pain via cold water immersion of their right hand as controls. The study aimed to investigate the immediate impact of rTMS on pain intensity. The results showed that the activation of the contralateral parietal cortex resulted in a reduction of pain intensity for up to 10 minutes. However, there was no discernible impact on pain in other cortical regions. Furthermore, the utilization of trains for three consecutive weeks on the contralateral parietal cortex did not yield enduring enhancements in pain thresholds (2).
Another clinical trial conducted by Ahmed et al. involved 27 patients who suffered from PLP due to unilateral amputation. Of the participants, 11 had undergone upper limb amputation, and 16 had undergone below-knee amputation. Over five consecutive days, a group of 17 patients received a 10-minute session of real rTMS targeting the hand region of motor cortex M1 on a daily basis. In contrast, a separate group of 10 patients received sham stimulation. Patients who underwent real rTMS exhibited a significant reduction in both Visual Analog Scale (VAS) and Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) scores compared to those who received sham rTMS. Furthermore, the Hamilton Scale demonstrated a significant reduction in depression and anxiety levels among patients who underwent real rTMS. After five sessions, beta-endorphin levels were evaluated in the 1 to 2 hours. The results indicated a significant increase in beta-endorphin levels following authentic stimulation, while no significant alteration was observed in patients who received sham rTMS. Therefore, it was concluded that the alleviation of pain could be attributed to an elevation in the levels of beta-endorphins in the serum (18).
A clinical trial by Malavera et al. included 54 patients. The study's findings indicated that the administration of active rTMS resulted in significantly higher pain relief 15 days after the treatment, as compared to sham stimulation. However, this effect was not found to be significant 30 days after treatment. Furthermore, 70.3% of participants in the active group experienced clinically significant pain relief (>30%), as compared to 40.7% in the sham group. The study also analyzed anxiety and depression symptomatology as a potential confounding factor in pain relief, revealing no significant differences between treatment effects groups over time (19). Bolognini et al. were the pioneering researchers to examine the impact of a single session of anodal or cathodal tDCS on the primary motor cortex (M1) and the posterior parietal cortex (PPC). The findings of their study revealed that the application of anodal stimulation to the M1 region resulted in an immediate and significant reduction of approximately 50% in PLP. Conversely, cathodal tDCS applied to the PPC produced similar but short-lived reductions, limited only to nonpainful phantom sensations. The initial study design failed to evaluate the enduring impacts of the tDCS treatment (16, 17).
In 2019, Kikkert et al. investigated whether targeting of missing hands would result in PLP relief. Excitatory tDCS was administered over the S1 (primary somatosensorial cortex)/M1 cortex of the missing hand, and the neural mechanisms underlying PLP relief were assessed during and after tDCS using neuroimaging techniques (14). The research conducted by Gunduz et al. in 2021 revealed that there were no significant enhancements in PLS. However, there was a significant improvement observed in PLP following the application of 10 sessions of 2 mA anodal tDCS for 20 minutes in conjunction with mirror therapy (13). Segal et al. revealed that there were no noteworthy enhancements in PLS. However, there was a significant improvement in PLP following the application of 10 sessions of 1.5 mA anodal tDCS for 20 minutes in conjunction with mirror therapy (12). A double-blinded, sham-controlled trial study was performed by Kikkert et al., which recorded brain activity with fMRI while stimulating M1 during phantom movements (14). Their study revealed that a decrease in sensorimotor cortex activity following stimulation was linked to a decrease in pain. Additionally, this research demonstrated that the reduction in cortical activity was preceded by modified activity in the mid-and posterior insula, as well as in the secondary somatosensory cortex (14). Table 1 presents a comprehensive examination of ten studies meticulously arranged in a sequential order based on their respective timelines.
Our study summarized the trials providing evidence regarding the effectiveness of anodal tDCS of M1 for PLP in comparison to sham stimulation in the immediate and short terms. It has been suggested that the modulation direction depends on the condition of the underlying network, and pain networks can be modified (88). Two notable limitations constrain this review. The varied frequencies and intervals of rTMS and tDCS employed in the studies hindered identifying the optimal parameters and session count for tDCS and rTMS in the management of PLP.
4. Conclusions
The current review demonstrated that there exists a moderate level of evidence to justify the utilization of tDCS and TMS in the treatment of PLP. Furthermore, no significant adverse effects were reported. Given that PLP is linked to maladaptive brain plasticity, interventions that regulate cortical reorganization and behavioral techniques, such as TMS and tDCS, respectively, may prove advantageous in managing pain associated with PLP.
Phantom limb pain has been demonstrated to be associated with cortical excitability within the M1 network. Furthermore, hyperexcitation of the posterior parietal cortex is demonstrated to be correlated with nonpainful phantom experiences. The heightened anodal excitability of tDCS in the primary motor cortex (M1) is associated with a reduction in PLP. Conversely, cathodal tDCS stimulation in the posterior parietal cortex is associated with an increase in non-painful sensations. rTMS has demonstrated potential as a viable treatment option for reducing pain sensation in PLP. This is supported by evidence demonstrating the correlation between pain scale measurements and the observed increase in serum beta-endorphin levels following treatment. To advance our understanding and application of neuromodulatory techniques for treating PLP, the next crucial step is the development of pragmatic trials. Such trials can assist in generalizing results to a wider population and assess critical outcomes pertinent to both patients and physicians, such as functional status and costs.
.png)