1. Background
Breast cancer is one of the top causes of cancer related mortalities among females. It was responsible for 2.1 million new cases and 626,679 cancer related deaths that were reported worldwide in 2018. Fair access to clinical and radiological assessment is crucial to early detection of breast cancer (1).
Although some guidelines and task forces have discarded clinical breast examination (CBE) (2), there is no doubt that CBE performed by appropriately trained breast physicians plays an important role in detecting breast cancer (3). Triple assessment ([CBE], imaging, and histo-pathological assessment) of breast pathologies is a very important method to detect breast cancer earlier.
The vast majority of palpable lumps are benign, but one cannot give %100 reassurance in terms of excluding malignant masses (4). Roughly, one-third of breast biopsies come back with diagnosis of breast cancer (5). This retrospective study aimed to ascertain the necessity of clinical-guided core biopsy (CGCB) or fine needle aspiration cytology (FNAC) for investigating clinically indeterminate breast lesions that have no significant imaging findings.
2. Objectives
The objective was to ascertain the necessity of clinical-guided core biopsy or fine-needle aspiration cytology for investigating clinically indeterminate breast lesions with no significant imaging findings.
3. Patients and Methods
We conducted a retrospective observational analysis of our current practice of 72 patients (who had undergone clinical core biopsy or FNAC) to investigate clinically palpable breast lesions that had normal imaging during the period from September 2017 to September 2019.
Based on our inclusion and exclusion criteria, we excluded 16 patients who were clinically graded as P4/P5, so the final number of patients included was 56 patients.
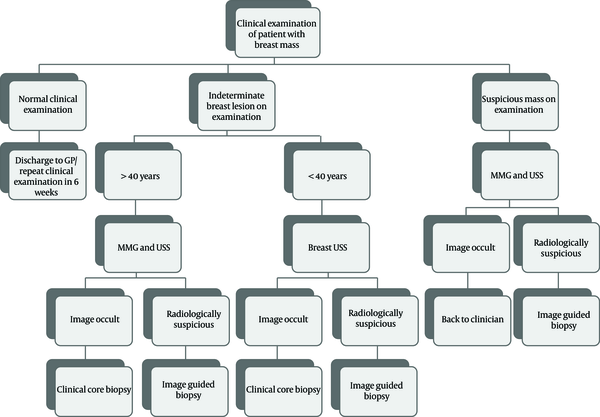
Clinical and radiological assessment pathway for breast care unit patients is demonstrated in Figure 1. Radiological examinations were carried out by four dedicated breast radiologists who are board certified from the United Kingdom (UK) (the first radiologist with more than 30 years of experience, the second and third with more than 15 years of experience, and the fourth, with two years of experience).
Ultrasound scans were carried out with the patient in the supine position, using two GE Logiq E9 stand alone machines, with transverse probes (ML6-15 MHz transducer) and elastography, color and power Doppler options.
Mammographic examinations were carried out in the standing position using GE Senographe EssentialTM digital mammography, and average breast thickness (60 mm, mAs 86.4, Kv 31, filter Rh [Rhadium], Target Rh).
Clinical core biopsies were performed by dedicated UK board certified breast surgeons, breast physicians and breast care nurses using 14 G AchieveTM core biopsy needle fully cocked technique.
Data was collected from the hospital clinic biopsy register, info-flex (Electronic patient records), and imaging and histopathology reports.
Clinical breast examination (CBE) and mammographic and ultrasound grading used for reporting was based on the UK 5-point breast imaging and reporting data system (UK BI-RADS) (R1: normal, R 2: benign 3: indeterminate /probably benign, R4: suspicious of malignancy and R5:highly suspicious of malignancy), which matched the 5-point clinical P grading system (U1, normal; U2, benign; U3, indeterminate/probably benign; U4, suspicious of malignancy; and U5, highly suspicious of malignancy), which matched the 5-point clinical P grading system (Table 1) (6, 7).
| Clinical | Ultrasound scan | Mammogram | MRI | Histopathology | Cytology |
|---|---|---|---|---|---|
| Px: Previous biopsy | |||||
| P1: Normal | U1: normal | R1: normal | MRI 1: normal | B1: normal | C1: normal |
| P2: Benign | U2: benign | R2: benign | MRI2: benign | B2: benign | C2: benign |
| P3: Indeterminate | U3: indeterminate | R3: indeterminate | MRI3: indeterminate | B3: indeterminate | C3: indeterminate |
| P4: Suspicious | U4: suspicious | R4: suspicious | MRI4: suspicious | B4: suspicious | C4: suspicious |
| P5: Highly suspicious | U5: malignant | R5: malignant | MRI5: malignant | B5a: non invasive cancer | C5: malignant |
| B5b: invasive cancer |
UK 5-Point Breast Clinical Examination and Imaging Classificationa
None of the 56 patients had post biopsy complications and all underwent written informed consent prior to the biopsy procedures.
4. Results
The total number of patients was 56 patients, the median age and mean age (standard deviation [SD]) were 46 years, and 48.3 years (14.8), respectively. Ten patients (17.8%) were younger than 40 years, while 46 patients (82.1%) were older than 40 years.
Fifty-five patients (98.2%) were female and only one patient (1.7%) was male.
The total number of examined breast lesions was 61 lesions, 27 patients (48.2%) had lesions on the right side, and 24 (42.8%) had left-sided lesions, while five (8.9%) had bilateral breast lesions.
Clinical assessment of index breast lesions was graded using P grading. All 61 included lesions were graded as P3.
Imaging modalities in the one-stop clinic included breast ultrasound as well as mammographic assessment, ultrasound scan was graded as U1 in 47 lesions (77%), U2 in 13 lesions (21.3%), and none were graded as U3, U4, and U5. One lesion (1.6%) did not have an ultrasound scan, as there was no suspicious lesion seen on mammogram.
Mammograms were not carried out for 20 patients (one male patient (1.7%), 10 patients (17.8%) younger than 40 years, and nine patients (16%) who had recent mammograms), 32 lesions (52.4%) were graded as R1 and nine lesions (14.7%) were graded as R2, none were graded as R3, R4, or R5.
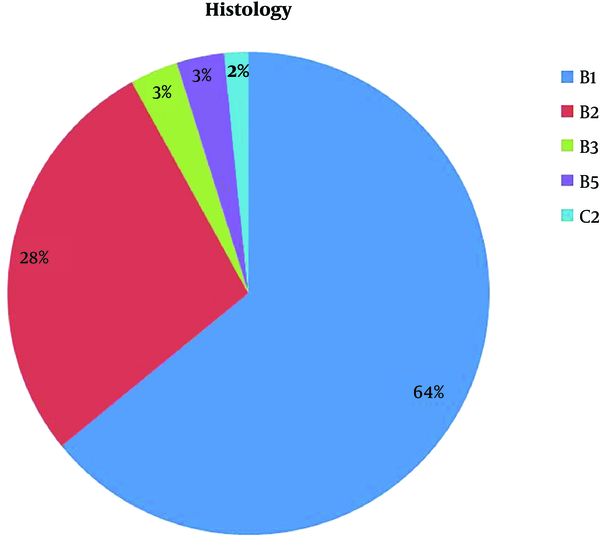
Histological assessment of clinical core biopsy revealed that 39 lesions (63.9%) were graded as B1, of which 17 were predominantly adipose tissue, and 22 were fibroglandular breast tissue.
Seventeen lesions (27.8%) were graded as B2, of which 10 were fibrous breast tissue, two fibrocystic disease, two chronic inflammation, one fat necrosis, one columnar cell change, and one pseudoangiomatous stromal hyperplasia (PASH).
Two lesions (3.2%) were graded as B3, one was atypical intraductal epithelial.
proliferation (AIDEP), and the other was flat epithelial atypia.
Two lesions (3.2%) were graded as B5 (one [1.6%] was found to be invasive lobular carcinoma [ILC], and the other one [1.6%] was found to be metastatic colorectal cancer to the breast). One lesion (1.6%) was investigated by FNAC; cytology was graded as C2 showing adipocytes in keeping with a lipoma (Figure 2).
5. Discussion
Breast cancer is the most common cancer in the UK, accounting for 15% of all newly diagnosed cancer cases per year. Fifty-five thousand women are diagnosed with breast cancer in the UK every year, (150 diagnosed every day).
The incidence of breast cancer is estimated to rise by 2% in the UK between 2014 and 2035. It may well reach to 210 cases per 100,000 females by 2035 (8). While a palpable breast lump is the most common presenting symptom of breast cancer, as reported by Mutar et al. (9) representing 71.3%, breast pain represents 18.9% of presenting symptoms.
Clinical breast examination usually precedes imaging for patients presenting to the breast clinic. Mutar et al. (9) and Koo et al. (10) found that patients usually demand breast examination if they experience a persistent lump other than other kinds of breast complaints.
Clinical breast examination is a simple non-invasive and cheap way to detect breast cancers, but adjunct imaging should be added to increase the final accuracy.
60% - 80% of breast biopsies usually show benign findings (11-13). Breast ultrasound and mammography could increase breast cancer detection rate by 4.2 cancers per 1000 screened women compared to women who undergo screening mammography alone (14).
Benson et al. reported the sensitivity of breast ultrasound as 89% (15). Houssami et al. (16) reported that the overall sensitivity of mammography and ultrasound is 96%.
The perception of a subtle mass or cluster of microcalcifications can be picked up by mammograms in case the image contrast is adjusted. Pisano et al. (17) and Yunus et al. (18) reported about 25% - 43% of non-palpable cancers can be detected on mammography in the presence of microcalcification.
In addition, Pisano et al. (19) found that the sensitivity and specificity of digital mammography in pre- or peri-menopausal women less than 50 years with non-dense breasts is 85% and 90%, respectively.
According to a study conducted by Dennis et al. (20), they followed up 540 breast lumps without ultrasound findings for at least 2 years, and they found that none of the lumps turned to be breast cancer or any other form of malignancy. This interesting result represents a negative predictive value of ultrasound is reaching 100%. Therefore, they advised ignoring clinical breast biopsy when imaging is negative (20).
According to Kaiser et al. (21), who conducted a prospective study with a patient cohort of 103, they followed up palpable breast thickening patients and reached a 100% negative predictive value of ultrasound imaging. Houssami et al. (22) reported that clinically guided biopsy (CGB) could have a high false negative rate compared to image-guided core or FNA sampling. Ward et al. (23) found that the diagnostic accuracy increases in case of image guided sampling.
Larger patient cohort studies have already demonstrated that core biopsy sampling approach has a better diagnostic outcome to FNAB in terms of sensitivity, specificity and correct histological grading (24-26).
Gumus et al. (27) advised that breast ultrasound scan should be performed for clinically palpable, mammographically occult breast lesions followed by clinically or ultrasound guided core biopsy.
The vast majority of our patients had a normal or benign findings on mammogram and ultrasound scans (Figures 3 and 4), yet clinicians opted to do a clinically guided needle biopsy to ascertain the final diagnosis.
In our patient cohort, we picked up two malignancies while Dennis et al. (20) and Kaiser et al. (21) reported none. One case was found to be ILC which is well known to be a hideous malignancy and can disguise itself even on MRI, while the second case was metastatic colorectal cancer to the breast. Therefore, any indeterminate breast lesions with negative imaging should be biopsied clinically, preferably with a core biopsy. The value of breast MRI scan can be arguable, it could be very valuable for most breast cancers, but hideous ILC or even invasive ductal carcinoma (IDC) can still be MRI negative (28).
Clinical assessment of palpable breast masses with normal imaging is challenging and harbour potential breast cancer misses. A no biopsy approach policy would result in potential delay in diagnosis. Ignoring the normal imaging findings and doing relentless CGB for all breast patients may result in too many unnecessary procedures.
Salzman et al. (29) introduced an algorithm/pathway for the management of patients presenting with breast lumps advising when to proceed with clinical guided biopsy and when to refer for imaging. In our breast unit, we have been using a similar algorithm with differences to meet our imaging guidelines (Figure 1).
Limitations of our study included, a small sample size, symptomatic patient cohort without screening patients, and clinicians’ and radiologists wide range of experience.
In conclusion, CGCB or FNAC are still valid approaches for investigating clinically indeterminate breast lesions with normal imaging. Multi-centric studies including a larger cohort of patients are still needed to come up with more robust results to deliver better patient care.