1. Background
In the days leading up to the end of 2019, new coronavirus was declared as the cause of coronavirus disease 2019 (COVID-19) responsible for the viral pneumonia outbreak in Wuhan, China (1). The Chinese official let the world know that a virus was spreading inside their communities, which then in the second week of February 2020, the international authorities responsible in such incidents, chose “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2) as the name for it. Accordingly, World Health Organization (WHO) recognized the related illness as “coronavirus disease 2019” (2). Ensuingly, it was determined that the current predicament is needed to be proclaimed as a pandemic (3). Appertaining to the WHO report on 11 June 2020, over 7.2 million individuals globally, have definite diagnosis of COVID-19 and the world’s death toll from the disease has currently surpassed 413000 which has resulted in a mortality rate of approximate 5.7 percent (4).
In Iran, first cases were reported in February 20, 2020. The severity of the situation was aggravated so quickly, that by 10th of March, Iran was amongst the five countries with the highest prevalence and death tolls because of COVID-19 (5) and by 11th June remained within the first ten countries (4). This condition had the Iranian National Health Service faced an increasing urgent demand for diagnosing infected cases, for the purpose of further isolation and medical care.
Based on the previous guidelines, COVID-19-induced-pneumonia diagnosis should be verified by reverse transcription polymerase chain reaction (RT-PCR) as benchmark (6). Given the restrictions occurring with collection of specimens and kit performance, the RT-PCR overall positive rate for samples collected by throat swabs has been described around 30% - 60% when initially presented (7). The preparation time for RT-PCR results to become available is variable and might be long in centers without in situ facility for analysis, which is also an important determining factor for clinical management. Apart from limitations of RT-PCR, with the uprising rate of suspected cases in an outbreak, the adequate number of kits would not be available as fast as required. Alternatively, computed tomography (CT) scan as a more accessible method, presents rapid results, and is available in many countries including Iran (8). Chest CT scan was suggested to be a satisfactory tool for detecting COVID-19 pneumonia based on reports from China, showing a sensitivity of up to 97% (9). A recent meta-analysis has demonstrated a pooled sensitivity of 94%; however, the positive predictive value of CT scan was questioned outside China where there is a low prevalence of disease (10).
At the same time, the radiation risk of mass CT examination must be taken into consideration, since young patients under 40 years old will require testing. In addition, follow-up CT examination might be required to monitor the disease progression with a reported mean of 4 ± 1 CT exams (ranging from 3 to 6) within a short interim of 4 ± 1 days (ranging from 1 to 8 days) (11). Thus, the cumulative radiation burden associated with repetitive CT examination is of concern.
Low-dose CT scan is approved for screening lung cancer with adequate quality (12). Encouraging data from a recent article demonstrated no breaks in double-strand-DNA with low-dose chest CT, as against standard-dose (13), particularly suggesting that the low-dose protocol is safer. A recent study has demonstrated high accuracy of low-dose chest CT in detection of COVID-19 (14). However, they have performed repeat RT-PCR tests in two consequent days, which is not easily available in many centers in our country.
2. Objectives
By conducting this research, we aimed to measure the accuracy of low-dose chest CT compared to first initial RT-PCR results, for detection of SARS-CoV-2.
3. Patients and Methods
3.1. Patients and Data Sources
The current cross-sectional study had been granted approval by the institutional review board (IRB), while the requisite to obtain patient consent has been waived. From February 20 to April 15, suspected COVID-19 patients, which had both chest CT scan and RT-PCR assay were enrolled in this single-center analysis.
The criteria for performing chest CT scan was based on the presence of one of the following symptoms according to our local hospital guideline: (1) Fever with a temperature exceeding 38° Celsius; (2) oxygen saturation of less than 93%; (3) respiratory rate higher than 30/min.
COVID-19 infection was confirmed with positive RT-PCR test, carried out by administering LightMix, SarbecoV E-gene RT-PCR Kits (Roche, Berlin, Germany). The results took a median time of 24 hours to be ready. Demographic variables (age, gender, and clinical symptoms), laboratory data (blood cell count, ESR, C-reactive protein, aspartate aminotransferase, alanine aminotransferase, blood urea nitrogen and creatinine) and initial RT-PCR test results were obtained from our hospital information system (HIS) providing electronic medical records of cases.
In patients with multiple chest CT scans, the initial scan was included in the study. Patients, in whom the interval between RT-PCR test and chest CT exceeded 7 days, were excluded from the study.
3.2. Chest CT Protocols
While the COVID-19 disease outbreak took place in Iran, our tertiary referral center, encountered daily rises in the number of chest CT scan requests. A remarkable number of cases were younger than 40 years old and according to the Iranian Radiology Society (ISR) consensus (8), we applied a low-dose chest CT protocol mainly adjusted from lung cancer screening CT protocol (15). Therefore, patients presenting in earlier days, underwent standard-dose CT scan; whereas, more recent cases underwent the low-dose protocol. Scanning parameters are presented in Table 1.
| Vendor | Activion 16, Canon | Somatom 16, Siemens | ||
|---|---|---|---|---|
| Protocol | Low-dose | Standard-dose | Low-dose | Standard-dose |
| Scan type | Helical | Helical | Spiral | Spiral |
| Rotation time, s | 0.75 | 0.75 | 0.6 | 0.6 |
| Detector configuration, mm | 16 × 1.0 | 16 × 1.0 | 16 × 1.2 | 16 × 1.2 |
| Pitch | Fast (1.438) | Standard (0.938) | 1.5 | 1 |
| kV | 120 | 120 | 110 | 130 |
| mA/reference mA | 30 | 80 - 500 | 20 | 110 |
| Sure exposure/CARE dose | ON | ON | ON | ON |
Standard-Dose and Low-Dose CT Protocols
Images were obtained with one of two CT scanners (Activion 16, Toshiba, Japan and Somatom scope power 16, Siemens Healthineers, Germany). The CT scan results were instantly transferred to picture archiving and communication system (PACS). Preliminary reports where available within 2 hours.
3.3. Radiation Dose Measurement
The volume of CT dose index (CTDIvol) in mGy, along with the dose-length product (DLP) in mGy.cm were obtained from the automatically generated dose report, based on the specific manufacturing company. Effective dose in millisievert (mSv) was computed by DLP × k formula, with k (the fraction of mSv over mGy.cm) assigned as 0.014 for chest CT according to the 2008 report of American Association of Physicists in Medicine (16).
3.4. Image Analysis
Two radiologists (B.S. and M.H. with 12 and 10 years experience, respectively in reporting chest CT scan) who were blinded to RT-PCR results and clinical symptoms, analyzed all images independently. They reported negative or positive CT, according to previous reports on typical and atypical CT findings of COVID-19 pneumonia (17). If the intended accordance was absent, both radiologists would carry out the read-out in concord.
3.5. Statistical Analysis
The statistical assessment was accomplished by employing version 21.0 of SPSS (IBM Corp. Released 2012. IBM SPSS Statistics for Windows, Armonk, NY). To display parameters, categorical variables were detailed as percentages and counts, and continuous variables were shown as mean ± standard deviation.
The accuracy, negative predictive value (NPV), positive predictive value (PPV), specificity, sensitivity, positive and negative likelihood ratio of low-dose chest CT were computed using RT-PCR outcomes as the reference. Comparison of quantitative data was performed using Student T test or Mann-Whitney U-test, according to the normal distribution assessed by Kolmogorov–Smirnov Z test. A P value of less than 0.05 was considered as statistical significance.
4. Results
4.1. Demographic Characteristics and Laboratory Data
Amongst 205 patients with both RT-PCR and CT scan results available, 42 patients had chest CT scan performed with standard-dose, acquired before the low-dose protocol was implemented or alternatively because COVID-19 pneumonia was not suspected at the initial presentation. A total of 163 patients with low-dose CT protocol were enrolled into the study (Figure 1) including 63 female (38.7%) and 100 male (61.3%) cases with the median age of 65 (ranging from 21 to 97) years. Among 163 patients, 28 (17.2%) were 40 years old or younger. All patients had one RT-PCR assay and at least one CT scan, with 16 patients with two or more CT scans. The mean interval between commencing of symptoms and RT-PCR testing was 4 ± 4 days, whereas the mean interval between commencing of symptoms and CT was 2 ± 2 days. Of 163 cases, 89 (54.6%) had positive results of RT-PCR for COVID-19. Within those 89 patients with confirmed diagnosis of COVID-19, more frequent clinical symptoms were dyspnea (75/89, 84.3%), fever (68/89, 76.4%), and cough (65/89, 73%). Demographic and laboratory results are presented in Table 2.
| Positive PCR (N = 89) | Negative PCR (N = 74) | P Value | |
|---|---|---|---|
| Ageb | 65 ± 18 | 57 ± 18 | 0.007 |
| Gender, Male, % | 53 (59.6) | 47 (63.5) | 0.631 |
| Clinical symptoms | |||
| Dyspnea, % | 75 (84.3) | 53 (71.6) | 0.057 |
| Fever, %b | 68 (76.4) | 44 (59.5) | 0.027 |
| Cough, %b | 65 (73) | 39 (52.7) | 0.009 |
| White blood cell count, G/L | 7.5 ± 3.4 | 7.7 ± 4.6 | 0.856 |
| Lymphocyte count, G/Lb | 0.98 ± 0.5 | 1.3 ± 0.6 | 0.002 |
| Lymphocyte percentage, %b | 15 ± 9 | 19 ± 10 | 0.006 |
| Erythrocyte sedimentation rate, mm/hb | 53 ± 36 | 22 ± 24 | 0.001 |
| C-reactive protein, mg/L | 54 ± 34 | 56 ± 39 | 0.844 |
| Aspartate aminotransferase, U/L | 64 ± 53 | 72 ± 82 | 0.510 |
| Alanine aminotransferase, U/L | 55 ± 36 | 70 ± 98 | 0.979 |
| Blood urea nitrogen, mg/dL | 61 ± 54 | 45 ± 26 | 0.146 |
| Creatinine, mg/dL | 1.5 ± 1 | 1.3 ± 0.6 | 0.515 |
Demographic and Laboratory Data of the Studya
4.2. CT Scan Findings
Of 163 patients, 151 (92.6%) had abnormal findings in CT scan at baseline. Among these 151 cases with abnormal CT, 133 (81.6%) were presented with typical CT findings of COVID-19 viral pneumonia assigned as positive. Most frequent findings included ground-glass opacity and consolidation mostly bilateral in peripheral/subpleural location (Table 3).
| Values | |
|---|---|
| Consistent with viral pneumonia (positive) | 133/163 (81.6%) |
| Findings | |
| Ground glass opacity | 122/133 (91.7%) |
| Crazy paving | 28/133 (21.1%) |
| Consolidation | 80/133 (60.2%) |
| Nodular infiltration | 7/133 (5.3%) |
| Reverse Halo | 2/133 (1.5%) |
| Lymphadenopathy | 4/133 (3%) |
| Pleural effusion | 6/133 (4.5%) |
| Distribution | |
| Peripheral/subpleural | 91/133 (68.4%) |
| Central/peribronchovascular | 5/133 (3.8%) |
| Both peripheral and central | 37/133 (27.8%) |
| Right lung | 3/133 (2.3%) |
| Left lung | 3/133 (2.3%) |
| Bilateral | 127/133 (95.5%) |
| Inconsistent with viral pneumonia or normal (negative) | 30/163 (18.4%) |
Chest CT Findings of 109 Patients with Low-Dose Protocol
Out of 163 patients, 18 (11.0%) had CT manifestations other than viral pneumonia including bacterial pneumonia, aspiration pneumonia (consolidation in dependent portion of the lung fields) or bronchiolitis (centrilobular nodular infiltration). Twelve patients (7.4%) had normal CT scan.
Compared to RT-PCR as the standard reference, the sensitivity, specificity, PPV, NPV, and accuracy of low-dose chest CT scan were 96.6% (95% confidence interval [CI], 90% - 99%), 36.5% (95% CI, 26% - 49%), 64.7% (95% CI, 56% - 73%), 90% (95% CI, 72% - 97%) and 69.3% (95% CI, 61% - 76%), respectively. Three cases with false negative CT results had normal CT scans. Positive and negative likelihood ratios were 1.52 (95% CI, 1.27 - 1.81) and 0.09 (95% CI, 0.02 - 0.29), respectively. Subgroup analysis was performed regarding patient’s gender and age, which are summarized in Table 4.
| Sensitivity, % | Specificity, % | PPV, % | NPV, % | Accuracy, % | PLR | NLR | |
|---|---|---|---|---|---|---|---|
| Value | 96.6 | 36.5 | 64.7 | 90 | 69.3 | 1.52 | 0.09 |
| 95% CI | 90 - 99 | 26 - 49 | 56 - 73 | 72 - 97 | 61 - 76 | 1.27 - 1.81 | 0.02 - 0.29 |
| Subgroups (n) | |||||||
| Male (n = 100) | 98.1 | 34 | 62.6 | 94.1 | 68 | 1.49 | 0.06 |
| Female (n = 63) | 94.4 | 40.7 | 68 | 84.6 | 71.4 | 1.59 | 0.14 |
| Age ≥ 60 (n = 94) | 100 | 36.1 | 71.6 | 100 | 75.5 | 1.57 | 0.00 |
Test Characteristics of Low-Dose Chest CT Scan for Detection of COVID-19 and Subgroup Analysis
The mean CTDIvol, DLP and effective dose of low-dose protocol were 1.77 ± 0.7 mGy, 64.7 ± 23.7 mGy.cm, and 0.91 ± 0.33 mSv, respectively. Overall, the radiation dose was remarkably lower than the observed amounts in 42 cases with standard-dose chest CT scan protocol (Figure 1), which were 12.8 ± 4.0 mGy, 413 ± 148 mGy.cm, 5.8 ± 2.1 mSv, respectively (P values < 0.001).
5. Discussion
Initial diagnosis of COVID-19 has been of essential significance in controlling the condition and providing medical care. Recent studies have demonstrated characteristic imaging findings in chest CT scan with promising accuracy in the detection of COVID-19 (17-19). Although the American College of Radiology proposes that chest CT must not be utilized for screening COVID-19 and it has to be reserved for hospitalized, symptomatic cases with specific clinical indications (20), in more epidemic areas including China, the local health officials have permitted the physicians to make the diagnosis based upon clinical data and chest CT findings (21) due to inadequate PCR kits in certain centers along with the considerable chance of false negative RT-PCR results. Also, a recent guideline from Fleischner Society has proposed that in epidemic regions with limited PCR test availability, an initial CT scan is indicated in suspected COVID-19 patients with moderate to severe symptoms for fast decision making (22). Similarly, the Iranian Society of Radiology suggested a low-dose chest CT protocol to carry out screening, especially in settings where the COVID-19 RT-PCR diagnostic kits are scarce (8).
Given RT-PCR outcomes as reference in 163 cases, we found a sensitivity and NPV of 96.6% and 90%, respectively, much the same as figures demonstrated by Ai et al. (9). In the mentioned study, a negative predictive value of 83% was indicated together with a sensitivity of 97%. Meanwhile, the average effective radiation dose was five to six times lower in this study compared to similar standard-dose chest CT exam.
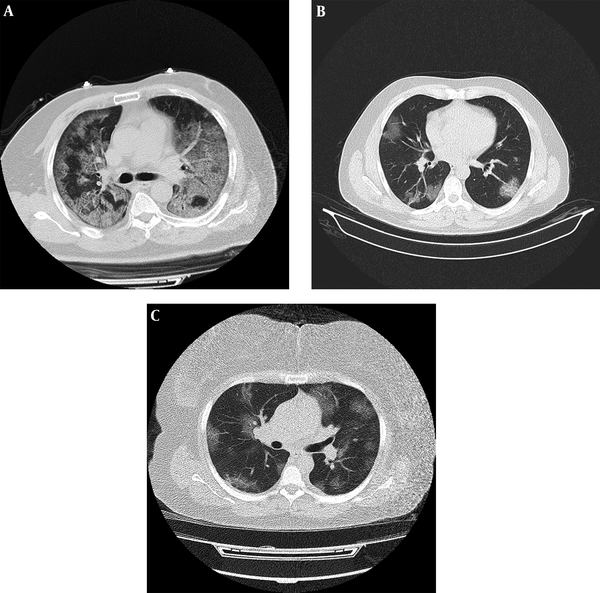
The low-dose chest CT protocol applied in this research has been modified from an existing lung cancer screening CT protocol, version 5.1, initially rendered by the American Association of Physicists in Medicine (AAPM). The CTDIvol average value in our study was 1.77 mGy, which is within the accepted limits for low-dose protocol in 16-row detector CT devices (15). The tube current–time product in our study was between 20 - 30 mAs with adequate quality of images (Figure 2). As described previously by Zhu et al. (23), comparison between the image quality of chest CT exams acquired at 25 mAs and those acquired at 115 mAs did not delineate substantial difference. Additionally, a higher percentage of images with normal-quality were seen in lung window setting compared to images with normal-quality in mediastinal window setting, which means better preserved quality in evaluating lung parenchymal abnormalities. Similarly, in the current study, even small peripheral patches of ground glass opacity were identified (Figure 3).
Examples of standard-dose and low-dose CT images with typical chest CT findings of COVID-19 pneumonia in patients with positive PCR. A, Fifty-seven years old male with fever and dyspnea for 10 days. Axial chest CT with standard-dose protocol (CTDIvol = 15.9 mGy) shows bilateral subpleural and peribronchovascular ground glass opacities and crazy-paving; B, Thirty-six years old male with fever and cough for 2 days. Axial chest CT with low-dose protocol (CTDIvol = 1.23) shows patchy bilateral peripheral ground glass opacities and early consolidation; C, Forty-eight years old female presenting with dyspnea for 4 days. Despite large body habitus of patient, axial chest CT with low-dose protocol (CTDIvol = 1.9) demonstrates multiple bilateral areas of peripheral and central ground glass opacities.
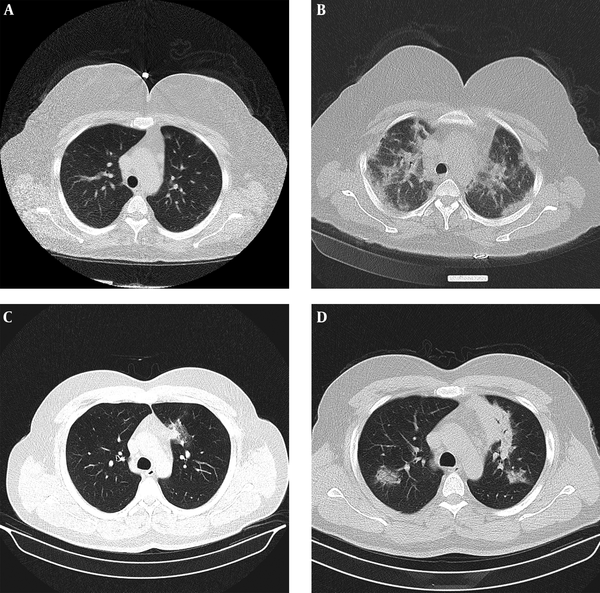
Progression of disease on serial studies. A, Axial chest CT with low-dose protocol (CTDIvol = 1.58 mGy) in 49 years old female with fever and dyspnea for 1 day shows small bilateral subpleural ground glass opacities; B, Axial chest CT 6 days later demonstrates progression of peripheral and central consolidation; C, Thirty-eight years old male with dyspnea for 6 days, axial chest CT with low-dose protocol (CTDIvol = 1.8 mGy) demonstrates single focus of ground glass opacity in paramediastinal portion of left upper lobe; D, Axial chest CT 4 days later shows increase in size and number of patches with more consolidate appearance.
We had 3/89 patients with normal CT scan and positive RT-PCR test. All three cases had CT scans acquired within 2 days from the symptom onset and this may be related to early imaging. As described previously by Bernheim et al. (24), from the cases who obtained CT scan within the first 48 hours after symptom onset, up to 56% had normal CT. Besides, Ling et al. (25) demonstrated that of 295 individuals with a confirmed diagnosis of COVID-19, 49 (17%) had a negative initial chest CT scan, while 34 (12%) remained negative after 3-14 days and mostly with few clinical symptoms.
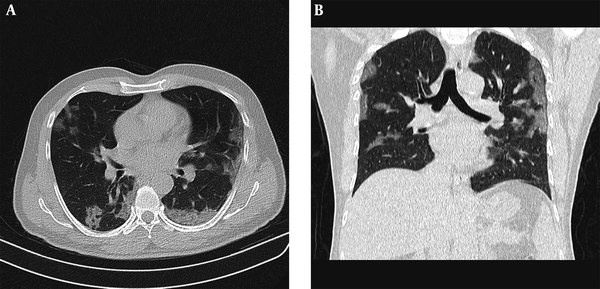
In our study, 63.5% (47/74) of patients with negative PCR results had typical CT findings of COVID-19, which is similar to a previous study by Ai et al. (9) reported as 70%. CT results of COVID-19 may be overlapped by findings in non-COVID pneumonia (26). Nonetheless, considering the rapid spread of COVID-19, what must be prioritized is to identify suspicious cases, so that early separation of affected individuals and their contacts, along with administration of proper clinical care could become possible. Also, it should be kept in mind that some of the false-positive patients on CT might in fact be true-positive, particularly when the RT-PCR’s low positive rate is taken into consideration (Figure 4) Accordingly, Long et al. (27) demonstrated that 6/36 patients with positive chest CT scan had a negative initial RT-PCR, which became positive after second and third repeats.
Example of positive chest CT scan findings for COVID-19 while negative PCR results. Fifty-four years old male with cough for 4 days, treated as COVID-19 according to clinical symptoms and CT findings. A, Axial; and B, Coronal chest CT with low-dose protocol (CTDIvol= 1.01 mGy) shows bilateral subpleural and peribronchovascular ground glass opacities.
A recent study by Dangis et al. (14) demonstrated a specificity of 93.6% for detection of COVID-19 with low-dose chest CT, markedly higher than results gathered in a recent meta-analysis by Kim et al. (10), which stated a pooled specificity of 35% (range 25% - 56%) from the studies with repeated PCR. Dangis et al. (14) claimed that their high accuracy was due to the repeat of RT-PCR test in the two following days; however, considering that previous studies with repeated RT-PCR results have not reached this high level of specificity, other possible explanations should be searched as well (28).
In the study by Dangis et al. (14), the accuracy of chest CT has not been compared to the first initial RT-PCR results, separately. In many hospital facilities in our country, there is relatively long delay for RT-PCR results to become available. We had a median waiting time of 24 hours in our center compared to few hours in the study by Dangis et al. (14). Hence, any repeat RT-PCR will double the time and become less practical from a clinical point of view.
Although it is important to finally prove COVID-19 infection with a positive PCR test, in our setting, with short supply to repeat RT-PCR, it was paramount for us to figure out the performance of low-dose CT compared to first initial PCR. We demonstrated the sensitivity and specificity of 96.6% and 36.5%, respectively which are in concordance with the values for standard-dose chest CT gathered in a recent meta-analysis by Kim et al. (10) as 94% for sensitivity (95% CI, 91% - 96%) and 37% (95% CI, 26% - 50%) for specificity.
Limitations in the current study include the following: First, we worked on a quite small quantity of cases. Second, in our study we could not directly compare low-dose CT accuracy with conventional CT, since there were only 42 patients who had both standard-dose CT and RT-PCR results in their profile. Therefore, we were not able to properly perform the non-inferiority test. Third, because of the shortage of RT-PCR kits, we could not repeat RT-PCR test, especially for negative cases, in the next following days. Therefore, we were not able to demonstrate that the results might eventually become positive somewhere down the process. Finally, we used RT-PCR tests as reference. Due to its rather small positive rate, the specificity of chest CT in SARS-CoV-2 cases might be understated; whereas, the sensitivity is overstated. Despite that, in an epidemic region, positive CT features, even with negative PCR results could still play a key clinical role in the rapid isolation of suspected cases for a better control of the viral spread. We believe that it is much needed to conduct further studies on this matter, due to its considerable clinical implication particularly in the low-resource settings where RT-PCR tests are not abundantly available for initial evaluation or to be repeated.
In conclusion, we have demonstrated that low-dose chest CT scan provides a high sensitivity and NPV in detecting COVID-19 pneumonia when compared to initial RT-PCR as the gold standard. Therefore, it might be considered as an alternate to standard-dose CT scan in epidemic areas with low availability of RT-PCR test.

.png)