1. Background
Using drug eluting stent (DES) is one of the most effective methods for acute myocardial infarction (1), but the effect has been seriously impaired by stent abnormalities (2). The mechanism is mediated by a series of vascular active substances, and the process could not be prevented easily (3, 4). Furthermore, the definite mechanism is not completely clear (5). The research by Lee et al. (6) showed that coronary artery calcification score and cardiac enzyme (CK-MB) could predict in-stent restenosis (ISR). Some scholars also noted that the distribution width of red blood cells reflecting the physiological state of the body could also predict ISR (7). Therefore, the occurrence of ISR may manifest itself in a variety of forms, and the indicators that reflect the physiological changes of the body may also reflect ISR. In this study, myocardial remodeling was used as a pathophysiological indicator (8).
2. Objectives
The main objective of this study was to analyze the possibility of myocardial blood flow index (MBFI) in the diagnosis of in-stent stenosis (ISS).
3. Patients and Methods
3.1. Clinical Protocols
Cases were continuously collected.
Inclusion criteria: Clinical follow-up data for 572 patients, who had been treated with DES for acute coronary syndrome, were being collected continuously from February 2008 to May 2015. There were 90 cases suspected of ISS. This prospective study was in conformity with the declaration of Helsinki. The approval number obtained from Medical Ethics Committee of Medical College of Wuhan University of Science and Technology was 202038. All patients gave written informed consent.
Exclusion criteria is listed in Figure 1.
3.2. Coronary Computed Tomography Angiography (CCTA) Protocol
3.2.1. Scanning Parameters
CCTA was performed using 256-slice multidetector computerized tomography (MDCT) scan (Brilliance, Philips Medical System, The Netherlands). The scan parameters included slice collimation = 128×0.625mm, rotation time = 0.27s, tube current-time product = 125 mAs, tube potential = 120 kVp and resulting CTDIvol = 10.5 mGy. The prospective electrocardiogram (ECG) gating scanner was used. Scanning was carried out at the end of deep inspiration. Based on the patient’s body weight, a bolus of 50 to 65 mL contrast media (Ultravist 370 mg iodine/mL, Bayer) was injected into the antecubital vein at the rate of 3.8 - 4.3 mL/s, followed by a 30ml bolus of saline solution chaser through a dual-head injector (Irich medical, Missouri XD2001, Germany). An automated bolus tracking method was used for triggering when the attenuation reached 120 HU in the region of interest of the ascending aorta with a 6 seconds delay. Prospective ECG-triggered sequential acquisition mode was performed in all patients (with the acquisition window of 30%-85% of the R-R interval). The maximum intensity projection (MIP), multi-plane reconstruction (MPR) and curved planar reconstructions (CPR) imaging were used to analyze.
3.2.2. CT Image Reconstruction
We reconstructed axial source images with a section thickness of 0.67 mm and a section interval of 0.33 mm by using a standard filtered back projection (FBP) algorithm with a high-resolution cardiac kernel/filter during the mid-diastolic phase. The center of the imaging window was set at 75% of the R-R interval.
3.2.3. Image Quality Assessment
Image quality was assessed by two senior specialists with 10 years working experience in CCTA, and the disagreement was resolved by discussing. Excellent images without artifacts or pixel noises were selected (9).
3.2.4. Data Analysis of CCTA
The visible lumen diameter was given as an average of the measurements on three representative axial slices using the electronic caliper tool provided with the scanner’s standard workstation (IntelliSpace Portal, Philips Healthcare) (10). The length and inner lumen diameter of the stents (including from Abbott Co, US, Boston Scientific Co, US, and Medtronic Inc, US) were 26 mm-30 mm and 2.8 mm - 3.5mm, respectively. The stent materials included cobalt and chromium alloy, platinum and chromium alloy, cobalt and nickel alloy, with the wall thickness less than 0.1mm and the profile of 0.95 mm - 1.19 mm. The drug film was sirolimus (Cypher, Jihnson & Johnson, USA).
Coronary artery plaque and stenosis were assessed by the fifteen-segment method (11). The new progressive plaque was judged by comparing with the result of previous invasive coronary angiography (ICA) before. When the stenosis was more obvious than before, it was defined as a new lesion.
3.3. Invasive Coronary Angiography Protocol
Invasive coronary angiography (ICA) was performed within 1 week after admission. The angiograms were reported by experienced invasive cardiologists blinded to the CCTA results. The ICA results were classified as follows: no stenosis; stenosis < 50%; 50% ≤ stenosis < 70%; 70% ≤ stenosis < 99%; and occlusion. Stenosis of the stent was classified as ISR with the lumen reduction ≥ 50%, or as stent neo-intimal hyperplasia (SNH) with the lumen reduction < 50%. The morphology of stent was evaluated by the literature (12), including focal stenosis < 10mm in the stent with a low density filling defect; diffuse stenosis ≥ 10mm in the stent with a low density filling defect; proliferative stenosis > 10mm and extending outside the either end of the stent with a low density filling defect; occlusion.
3.4. Myocardial Blood Flow Index (MBFI)
3.4.1. Myocardial Blood Flow Model
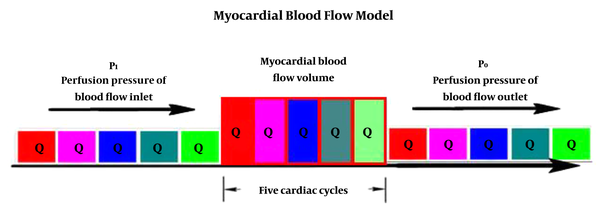
According to the lumped parameter network (LPN) (13), myocardial perfusion was assumed to be a parallel impedance model (14), and myocardial blood flow was mainly determined by the ratio of myocardial blood perfusion pressure to myocardial microcirculation resistance (myocardial mass) (15). Since the cardiac blood circulation is pulsed from the artery end to the vein end in about five cardiac cycles (16), that is, after five cardiac cycles, a certain quantity of blood is distributed to the whole cardiac microcirculation. Therefore, the ratio of myocardial blood flow quantity to the mass is the myocardial blood flow (Figure 2). Because body mass index (BMI) (17), gender (18), and age (19) all correlate with coronary artery disease, so MBFI should be corrected.
3.4.2. Calculation of MBFI
Using Omron upper-arm blood pressure monitors (Omron U10K, Dalian, China), the patients’ diastolic blood pressures (DP) were measured in a quiet condition, and the heart rates (HR) were recorded three times after a 10-minute interval in the sitting position. Then, using the post-processing software, the myocardial mass (M) was calculated. The cardiac cycle (time, T) was calculated as 60 seconds divided by HR within one minute. The myocardial perfusion time (PT) was equal to 5 multiplied by T. As a result, MBFI = PT multiplied by DP/ (M multiplied by BMI) (14, 16, 20).
3.5. Diagnostic Analysis
ICA was used to define the degree of ISS: SNH with ISS < 50%, and ISR with ISS ≥ 50%. The receiver operating characteristic (ROC) curve was analyzed by 2 times the standard deviation of the mean, and the optimal cutoff value of MBFI was determined to diagnose the stent abnormality. The diagnosis accuracy was analyzed, including the sensitivity, specificity, positive predictive value, and negative predictive value.
3.6. Statistical Analysis
Statistical analyses were performed using commercially available software (MedCalc Software 18.2.1, Ostend, Belgium). Quantitative variables were expressed as mean ± standard deviation (SD), and categorical data were given in proportions and percentages. Continuous variables were compared using t test, whereas count data were compared using chi-square test. P < 0.05 was considered statistically significant.
Multivariate regression analysis was performed to obtain the weight coefficient of the old age. MBFI and old age were assumed to be high risk factors for ISS. Old age was considered as higher or equal to 60 years for male or higher or equal to 55 years for female. The age intervals were set according to an interval of 5 years. The contribution of old age in the model was multiplying the intervals by the weight coefficient of age.
4. Results
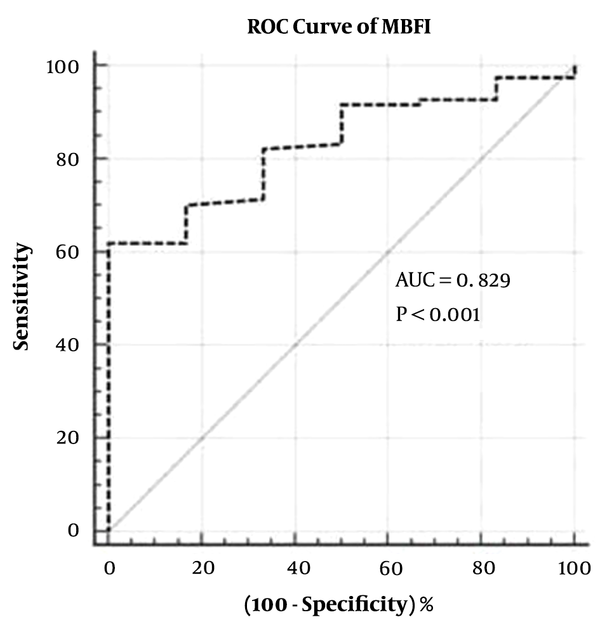
Among the 90 patients (mean age, 62.6 ± 10.02 years), the time of stent implantation was 7.1 ± 4.51 years. Baseline characteristics are shown in Table 1. MBFI was the main risk factors of ISS (P < 0.0001). The weight coefficient of the old age was 0.01. Corrected MBFI = MBFI -k multiplied by n (k = 0.01 (the weight coefficient)), n = age intervals. Two times the standard deviation of the mean value was 0.100, and the optimal cut-off value was 0.082 (area under the curve [AUC] = 0.829, 95% CI: 0.736 - 0.900) (Figure 3). There were 39 cases with MBFI ≤ 0.082, of which 34 (37.8%, 34/90) showed ISS, including 18 (20%, 18/90) with SNH, 16 (17.8%, 16/90) with ISR (Figure 4). In the 39 cases with MBFI ≤ 0.082, 8 (20.5%, 8/39) presented new lesions, and 7 (17.9%, 7/39) presented severe lesions. There were 51 cases with MBFI > 0.082, and only one case with SNH (Figure 5). The sensitivity, specificity, positive predictive value, and negative predictive value were 91.4%, 89.1%, 84.2%, and 94.2%, respectively, and the accuracy was 90.0%.
| Characteristics | Male (n = 59) | Female (n = 31) | t or χ2 value | P |
|---|---|---|---|---|
| Age, y | 64.8 ± 9.62 | 63.6 ± 8.32 | -0.588 | 0.5579 |
| BMI, kg/m2 | 24.2 ± 4.12 | 25.2 ± 2.69 | 1.220 | 0.2257 |
| Heart rate, times/min | 71.7 ± 10.10 | 70.5 ± 10.02 | -0.537 | 0.5926 |
| Diastolic pressure, mmHg | 77.8 ± 11.12 | 75.3 ± 13.15 | -0.951 | 0.3442 |
| Perfusion time, s | 4.2 ± 0.79 | 4.3 ± 0.55 | 0.629 | 0.5313 |
| Time of stent implantation, y | 7.5 ± 4.23 | 7.1 ± 4.55 | -0.415 | 0.6789 |
| In-stent stenosis, n | 23 | 11 | 0.105 | 0.7463 |
Characteristics of the Study Populationa
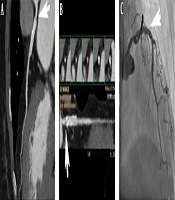
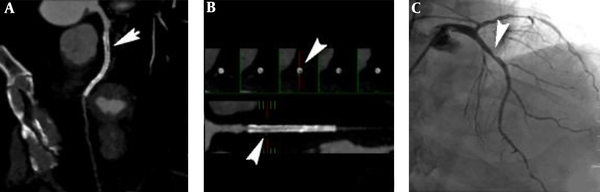
A 70-year-old female patient with unstable angina, left anterior descending (LAD) artery, and two drug eluding stents (DES) for the diffuse severe lesions. A and B, Computed coronary tomography angiography (CCTA) shows an in-stent restenosis (ISR) (arrows, window level 486, window width 843) in the LAD artery with the myocardial blood flow index (MBFI) of 0.055; C, Invasive coronary angiography (ICA) shows a stenosis degree of 70% in the stent (arrow).
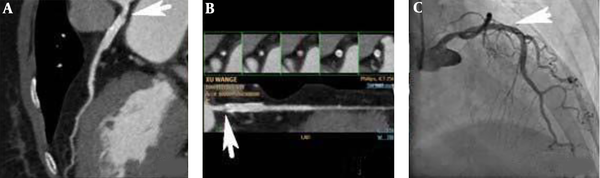
A 78-year-old male patient with unstable angina, left anterior descending (LAD) artery, and a drug eluding stent (DES) for the local severe lesion. A and B, Computed coronary tomography angiography (CCTA) shows a small low-density shadow (arrows, window level 453, window width 872) in the proximal segment of stent with the myocardial blood flow index (MBFI) of 0.089; C, Invasive coronary angiography (ICA) does not show in-stent stenosis (ISS) in the stent (arrow).
5. Discussion
Cardiac remodeling, as an important aspect of pathological changes in coronary artery disease (CAD) (21), would be an important breakthrough point for the study of ISR. In this study, with the ICA as the gold standard, among all 39 cases with MBFI ≤ 0.082, there were 34 cases with ISS (37.8%, 34/90), including 18 cases (20.0%, 18/90) of SNH, 16 cases (17.8%, 16/90) of ISR, which was higher than the literature (< 10%) (22). There were a few reasons for the higher incidence rate of ISR including enrolled cases with the vast majority of older patients (75.6%, 68/90), the relative long time after stent implantation (up to 13 years), and the different type of DES with the inconsistent efficacy to prevent ISS (23). It can be seen that stent abnormalities include SNH and ISR (24), which can be analyzed by MBFI. To know whether MBFI can further differentiate SNH from ISR directly, further studies are necessary.
For CCTA, evaluating ISS is susceptible to some factors, such as metal artifacts, respiratory movement artifacts, arrhythmia, and the limited spatial resolution. It could be difficult for CCTA to evaluate ISS accurately. If a special tool is used in the diagnosis of ISS, the accuracy would improve obviously. This study used MBFI to evaluate ISS without thinking about the weakness of CCTA, and found out that the accuracy was satisfied with MBFI, and the sensitivity, specificity, positive predictive value, negative predictive value were 91.4%, 89.1%, 84.2%, 94.2% respectively, and the accuracy was 90%. The introduction of MBFI to analyze stent abnormalities in the pathophysiological perspective of remodeling could avoid the method of conventional deficiency based on the morphology of the stent, and provide an objective basis to prevent ISR in a high-risk population with an ISS lower than 50%.
After stent implantation, patients were treated with dual-antiplatelet therapy (aspirin combined with clopidogrel) and secondary prevention of CAD in accordance with relevant guidelines (25), and the plaque of the coronary artery should be stable or improved. However, in this study, in the 39 cases with MBFI ≤ 0.082, 20.5% (8/39) were found with the new-onset plaque and 17.9% (7/39) presented with severe stenosis after stent implantation. The reasons could be remodeling factors including cytokines inducing smooth muscle cell differentiation, accelerating cell synthesis, and promoting proliferation and migration, which could induce the occurrence of the unstable atherosclerotic plaque. They could be the potential risk factors leading to acute coronary syndrome and great attention should be paid to them in clinical practice.
This study has limitations. First, this study chose a resting state in a relatively short period of one day, so further research is needed to determine which time is more representative. Second, similar to the risk factors of the elderly, multi-stent implantation, diabetes and multi-vessel lesions are also risk factors of ISS, so it is necessary to further explore their roles in the model.
In conclusion, all patients with reduced MBFI values after stent implantation should be actively prevented from the occurrence of ISR, and ICA examination should be performed if necessary. Second, ISS could accompany new severe lesions leading to acute coronary syndrome, to which great attention should be paid in clinical practice.