1. Context
An aortic aneurysm is a peripheral enlargement or a bulging of the wall of the aorta. It occurs when a part of the aortic wall dilates and forms a bulge, or when the aortic wall diameter is at least 1.5 times larger than normal, appearing tube-shaped (fusiform) or round-shaped (saccular). An aortic aneurysm can occur anywhere in the aorta. An abdominal aortic aneurysm (AAA) occurs along a part of the aorta that passes through the abdomen (1, 2).
AAA is considered the 14th leading cause of mortality in the USA (3). Also, 4500 reported deaths are related to AAAs annually, and 1400 deaths due to 45,000 repair procedures are performed to prevent aortic ruptures (4). Clinically, the prevalence of aneurysms (> 4 cm in diameter) in men, aged 55 - 64 years, is about 1%; after this age range, the prevalence rate increases by 2% to 4% with each decade (5, 6). AAA is also four times more common in men than women (7, 8); however, it occurs in women about 10 years later than men (9). Besides, a previous study found that AAA was more common in white people than in black people (10).
The risk factors for AAAs include age, sex, race, and smoking. The risk of AAA increases significantly after the age of 60 years (5, 6). Also, the risk of aneurysm rupture is affected by several factors, including the aneurysm size, rate of dilation, and sex (11). The smaller the AAA diameter is, the lower the risk of rupture will be. In other words, for diameters < 4 cm, the rupture risk is negligible; for diameters of 4 cm - 4.9 cm, the rupture risk is only 0.5% - 5% each year; and for aneurysms with diameters of 5 cm - 7 cm and 7 cm - 8 cm, the rupture risk is 3% - 20% and 20% - 40% each year, respectively (11). Generally, the AAA rupture is associated with a high mortality rate (12). Only 50% of patients, in case of a ruptured AAA, arrive at the hospital alive, up to 50% of whom do not survive the recovery process (13); therefore, the overall mortality rate is estimated at 80% (14).
The majority of AAA patients are asymptomatic, and most diagnoses are accidental (12). AAA is treated by open surgery or endovascular interventions. Since the introduction of endovascular aneurysm repair (EVAR) in the 1990’s, the difference in the cost-effectiveness of this procedure versus open surgical repair (OSR) has been considered (15). The present study aimed to systematically evaluate the cost-effectiveness of EVAR versus OSR for AAA ruptures.
2. Methods
2.1. Search Strategy
This systematic literature review was carried out for the economic assessment of EVAR versus OSR for ruptured AAAs during 1999 - 2020. A search was performed in PubMed, Medline, Embase, Web of Science (ISI), National Health Service Economic Evaluation Database (NHSEED), Database of Abstracts of Reviews of Effects (DARE), Tufts Medical Center Cost-Effectiveness Analysis Registry, Health Technology Assessment (HTA) database, Scopus, Cochrane Library, Institute for Clinical and Economic Review (ICER), and National Institute for Health and Care Excellence (NICE).
To identify cost-effectiveness analysis studies comparing EVAR with OSR, the following Medical Subject Heading (MeSH) terms were searched and combined: “Abdominal aortic aneurysm”, “endovascular”, “open repair”, “rupture”, “economic evaluation”, and “cost-effectiveness”. Besides, the reference lists of articles were reviewed to find further articles. The results were reported based on the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) guidelines (16, 17). This study was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database of York University, UK (PROSPERO registration code: RD42018088472).
2.2. Study Selection
Initially, duplicates were removed from our search list by screening the titles and abstracts of the reviewed articles. Each article was then reviewed to find content related to the treatment of AAA ruptures and economic assessment. Next, the selected articles were reviewed based on the inclusion and exclusion criteria and screened for further analysis. The articles were retrieved by two reviewers, and the results were compared. Agreement was reached for any discrepancy between the two reviewers through discussion with a third reviewer.
2.3. Inclusion and Exclusion Criteria
The following factors were considered as the inclusion criteria in this review: (1) Study designs, including complete health economic evaluation models (i.e., cost-effectiveness analysis, cost-utility analysis, and cost-benefit analysis); (2) study populations, including patients with ruptured AAAs; (3) interventions, including EVAR; (4) comparators, including OSR; and (5) outcomes, including quality of life (QALY), life years gained (LYG), and any outcomes for economic evaluation, such as the incremental cost-effectiveness ratio (ICER) and cost-effectiveness.
On the other hand, the following factors were considered as the exclusion criteria: (1) Partial economic evaluation studies, such as cost-saving analysis (CSA) or cost analysis (CA); (2) non-English language studies; (3) summaries, short reports, commentaries, letters, protocols, CSAs, conference abstracts, case reports or case series, letters, comments, editorials, or review articles; (4) unavailable full-text of the article; and (5) published studies before 1999.
2.4. Quality Assessment
By using the Quality of Health Economic studies (QHES) checklist, the methodological quality of the selected studies was evaluated. The QHES is one of the preferred checklists in economic evaluation studies, which assesses both quantification and reporting standards, based on the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist (18-20). The QALY, LYG, and ICER were considered as the main outcomes of this review, which focused on comparing the use of EVAR with OSR for ruptured AAAs (20, 21).
2.5. Data Extraction and Analysis
In the present study, the search strategy was strictly based on the inclusion and exclusion criteria to find relevant articles. The main study specifications were as follows: the first author’s name, country and year of study, number of patients, mean age of patients, study model, study perspective, reported outcomes, QALY, time horizon, type of sensitivity analysis, discount rate, cost analysis, and ICER in each study. Articles were evaluated with data extracted critically. Two authors independently extracted the relevant information. A qualitative analysis was also performed to abstract the results of studies, considering the uncertainties and heterogeneity between different studies.
3. Results
3.1. Literature Search Results
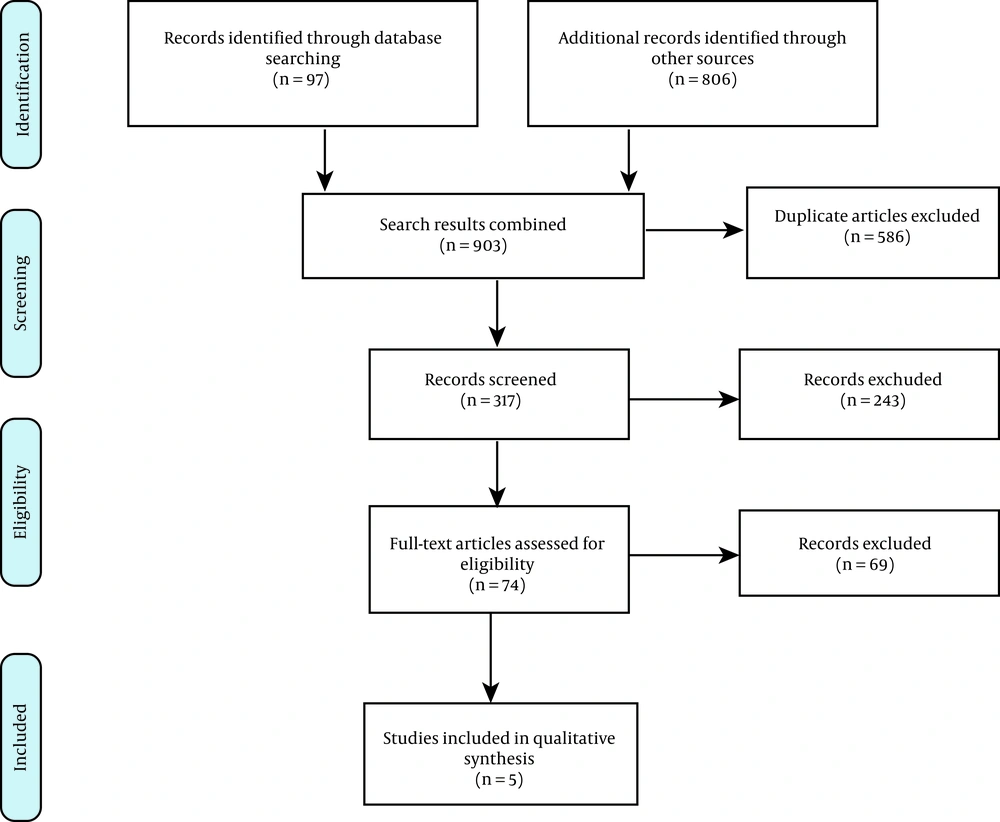
Our initial search yielded 903 potentially relevant citations, 97 of which were retrieved from PubMed and 806 from other sources. After removing 586 duplicates, the titles and abstracts of the remaining 317 articles were reviewed. Also, 243 studies were removed because they did not meet the inclusion criteria. Next, 74 studies were reviewed for their full-text abstracts, and 69 were removed based on the exclusion criteria, insufficient reports, or absence of an appropriate method evaluated by the QHES checklist. Finally, the remaining five studies were considered appropriate to analyze the cost-effectiveness data and the characteristics of studies. Figure 1 presents our study selection strategy, based on the PRISMA guidelines.
3.2. Characteristics of Studies
The general characteristics of the retrieved articles are presented in Table 1. Studies were performed in European countries, including the United Kingdom, the Netherlands, and Ireland. Three of the studies were conducted in the UK (22-24), one was performed in the Netherlands (25), and one was carried out in Ireland (26). In most studies, the populations were slightly different, and in all studies, the diameter of AAAs was larger than 5.5 cm. Regarding the patients’ age, except for one study (25), the age of the population was above 70 years in other studies.
| Authors’ names and year of publication | Country | Patient number | Population | Mean age, y | Perspective of the study | Time horizon, y | Health outcomes | Sensitivity analysis | Discount rate |
|---|---|---|---|---|---|---|---|---|---|
| Hayes et al. (22), 2010 | United Kingdom | 730 | AAA > 5.5 cm | 70 | Third-party payer (UK NHS) | 30 | Incremental costs per QALY gained | One-way and multivariate sensitivity analyses | - |
| Kapma et al. (25), 2014 | The Netherlands | 116 | Based on the AJAX trial | - | Third-party payer | 2 | Incremental costs per QALY gained | one-way & probabilistic sensitivity analysis | - |
| Rollins et al. (23), 2014 | United Kingdom | EVAR: 62; OSR:85 | Based on the IMPROVE trial | EVAR:77.9; OSR:75 | Third-party payer (UK NHS) | 3 | Incremental costs per QALY gained | - | - |
| Powell (24), 2017 | United Kingdom | 316 | AAA > 8 cm | 76 | Third-party payer | 3 | Incremental costs per QALY gained | Probabilistic sensitivity analysis | 3.5% |
| Canning et al. (26), 2019 | Ireland | 88 | Based on the IMPROVE and AJAX trials and AAA > 7 cm | EVAR:72; OSR:73 | Third-party payer | 3 | Incremental costs per QALY gained | - | - |
Abbreviations: AAA, Abdominal aortic aneurysm; AJAX, Amsterdam Acute Aneurysm, EVAR, endovascular aneurysm repair; NHS, National Health services, OSR, open surgical repair; QALY, quality-adjusted-life year.
In a study by Hayes et al. (22), the largest patient population was 730 patients, while other studies had different population sizes, ranging from 60 to 316 patients. The perspective of the selected studies was not significantly different, as in all of the studies, it was a third-party payer’s perspective. The time horizon was three years in nearly all of the studies (23, 24, 26). Nevertheless, one study had a time horizon of 30 years (22), and one study had a time horizon of two years (25). The discount rate was not mentioned in most studies, except for one study that reported a 3.5% discount rate (24). In the study by Hayes et al. (22), a combination of decision tree and Markov model was used for cost-effectiveness modeling, while the trial type was mentioned in only one study (24); other studies did not specify the model applied.
The effectiveness of the intervention was evaluated by QALYs in all studies. With the exception of two studies (23, 26), other studies performed a sensitivity analysis to determine the effect of ICER on the input variables. One of these studies conducted one-way and multivariate sensitivity analyses (22); one study used one-way and probabilistic sensitivity analyses (25); and one study only performed a probabilistic sensitivity analysis (24). The ICER in the selected studies was reported in different proportions and ratios. The EVAR intervention was dominant in one study, without requiring the ICER calculation (24).
Although EVAR was not introduced as a cost-effective approach in one of the studies (23), the rest of the studies found it to be cost-effective (22, 23, 26). The findings of our study showed that all selected studies had considered the direct medical costs, including hospital admission, follow-up (outpatient appointments), cost of surgery, operating room equipment, blood products, diagnostic procedures (e.g., pathology), consultation, dialysis, physiotherapy, imaging procedures (e.g., duplex CT imaging), and Intensive Care Unit (ICU) or Ward Stay. However, one study did not define the exact costs (Table 2) (24).
| Study/Citation | Price year | Study model | Type of cost | Threshold | Incremental costs | Incremental QALYs /LY gained | Incremental cost per QALY gained (ICER) | Is EVAR cost-effective? |
|---|---|---|---|---|---|---|---|---|
| Hayes et al. (22), 2010 | 2010(£) | Decision tree and Markov model | Length of hospital stay, ICU stay, and surgery time | £20,000 - £30,000 | -£1508 ($2262) | 0.64 | £-2,359 | Yes |
| Kapma et al. (25), 2014 | 2010 (€) | - | Direct and indirect medical costs: Surgery, equipment, blood products, hospital admission, diagnostics, and others (e.g., pathology, consultation, dialysis, and physiotherapy) | €80,000 | 30 day: €5,306; 6 months: €10,189 | 0.026 | 30 day: €120,591 6 months: €424,542 | No |
| Rollins et al. (23), 2014 | 2013 (€) | - | Direct medical costs: Surgery, equipment, blood products, hospital admission, and follow-up (outpatient appointments, CT scan, duplex imaging, ICU stay, and ward stay) | - | €-2,027 | 0.43 | €-4933/QALY | Yes |
| Powell (24), 2017 | 2013 (£) | Trial | - | £30,000 | £-2,605 | 0.166/0.115 | D+ | Yes |
| Canning et al. (26), 2019 | 2016 (€) | - | Direct medical costs: Operating room, equipment, and hospital admission | - | 2,858 | 0.122 | €23,426 | The IMPROVE trial found EVAR to be cost-effective, and the AJAX trial found EVAR to be unaffordable. |
Abbreviations: AJAX, Amsterdam Acute Aneurysm; D+, EVAR dominates OSR (EVAR is more effective and less expensive); EVAR, endovascular aneurysm repair; ICER, Incremental cost-effectiveness ratio; OSR, Open surgical repair.
3.3. Quality assessment of studies
The quality assessment of five studies, based on five questions in the QHES checklist, is presented in Table 3. Each question in the QHES checklist has a specific weight and scoring method (from 0 to 100). Studies were divided into one of the following four categories, based on the obtained score: 0 - 24, poor quality; 25 - 50, low quality; 51 - 74, good or average quality; and 75 - 100, excellent quality. According to the results, all studies met the checklist items. One study showed an average quality (26), while other studies were found to have excellent quality.
| Item | Study | Hayes et al. (22), 2010 | Kapma et al. (25), 2014 | Rollins et al. (23), 2014 | Powell (24), 2017 | Canning et al. (26), 2019 |
|---|---|---|---|---|---|---|
| 1 | Study objective | Y | Y | Y | Y | Y |
| 2 | Perspective | Y | Y | Y | Y | Y |
| 3 | Study design | Y | Y | Y | Y | Y |
| 4 | Subgroup analysis | Y | Y | Y | Y | Y |
| 5 | Sensitivity analysis | Y | Y | N | Y | N |
| 6 | ICER between alternatives | Y | Y | Y | Y | Y |
| 7 | Data abstraction | Y | Y | Y | Y | Y |
| 8 | Discount | N | N | N | Y | N |
| 9 | Cost measurement | Y | Y | Y | Y | Y |
| 10 | Economic outcomes | Y | Y | Y | Y | Y |
| 11 | Health outcome reliability | Y | Y | Y | Y | Y |
| 12 | Calculation procedure | Y | Y | Y | Y | Y |
| 13 | Limitations | Y | Y | Y | Y | N |
| 14 | Potential bias | N | N | Y | N | N |
| 15 | Conclusion | Y | Y | Y | Y | Y |
| 16 | Funding | Y | N | N | Y | N |
| 87% | 84% | 81% | 94% | 71% |
Abbreviations: QHES, quality of health economic studies; ICER, incremental cost-effectiveness ratio.
4. Discussion
This study is the first systematic review that comprehensively evaluates the cost-effectiveness of OSR for AAA ruptures versus EVAR. We systematically reviewed the results of five validated studies and concluded that EVAR could be a more cost-effective intervention than OSR for patients with ruptured AAA.
In a study by Hayes et al. with a 30-year time horizon (22), the average cost was $26,133 for EVAR and $28,395 for OSR. Also, the quality of life of patients undergoing EVAR was higher than that of OSR patients (3.09 vs. 2.04). According to the results of this study, EVAR improves the short-term survival rate and reduces the length of stay in the ICU compared to OSR. No short-term complications occurred in the OSR group, and treatment costs showed no change in this group. The researchers concluded that at a threshold of 30,000 - 45,000/QALY, EVAR could be considered a cost-effective method.
Different variables can affect the cost-effectiveness outcomes of EVAR compared to OSR. The results of a sensitivity analysis by Hayes et al. (22) showed that some parameters, such as the length of stay in the ICU, transfer of blood products, and cost of EVAR device, contribute to cost-effectiveness. Besides, the most important factor affecting the ICER was the length of stay in the hospital or ICU. On the other hand, a study by Kapma et al. (25), by performing a one-way probabilistic sensitivity analysis, showed that the most important factor in EVAR was the cost of stent. In other words, if the cost of stent reduced by 25%, the total direct medical costs could be €30,768 and €39,377 during 30 days and six months, respectively, leading to differences of €3,332 and €8,215 versus OSR, respectively. Also, if the cost of stent reduced by 50%, the direct medical cost of EVAR would reach €1,470 in 30 days and € 6,354 in six months. The results of this study showed that at a threshold of €80,000, the cost-effectiveness of EVAR was 25%.
In this systematic review, the included studies used different types of data. For example, in a study by Powell (24), an IMPROVE trial, with a maximum follow-up period of 7.1 years, reported an average hospital stay of 14.4 days in the EVAR group and 20.5 days in the OSR group. Also, the patients' quality of life improved over three years in the EVAR group, with no increase in the re-intervention. The cost of EVAR was £16,878, and the cost of OSR was £19,483; the incremental net benefit was -£7,637. At a willingness to pay of £30,000, EVAR is probably 90% more cost-effective.
In a study by Kapma et al. (25), the researchers used the data of Amsterdam Acute Aneurysm (AJAX) trial and found that the six-month EVAR mortality rate was lower than that of OSR. The 30-day mortality rate was 21% for EVAR and 25% for OSR, while at six months, the corresponding rate was 28% for EVAR versus 31% for OSR. Although in this study, EVAR was more effective than OSR, due to its higher cost, it could not be considered a cost-effective option with respect to the level of people’s willingness to pay. During six months, the average total cost was €41,350 for EVAR and €31,161 for OSR. The average cost difference between the two groups was €5,306 over 30 days and €10,189 over six months. However, no significant difference was found between the two groups in terms of quality of life.
In a study by Canning et al. (26), the data of both AJAX and IMPROVE clinical trials were examined. Based on the AJAX trial, the endovascular intervention was not cost-effective, whereas it was considered cost-effective in the IMPROVE trial. However, in these two trials, the quality of life of the endovascular group was higher than that of the OSR group (1.67 vs. 1.54). Since the indirect costs and direct non-medical costs were not investigated in the selected studies, there were uncertainties in evaluating the economic costs of these interventions. Obviously, this disease imposes indirect costs on the patients and their families, which increases the treatment costs.
Overall, generalization of the present results should be avoided due to some limitations, such as cost heterogeneity between different countries and healthcare systems, which is an inherent feature of economic evaluation studies; lack of sufficient long-term economic evaluation studies; different time horizons; and different follow-up periods. Therefore, researchers must conduct further studies according to local standards to consider the characteristics of each healthcare system, as well as the conditions of each country, while generalizing and using the results.
Regarding the crisis of inadequate health resources around the world, evidence-based decision-making is more necessary than ever. Economic evaluation of health interventions, especially cost-effectiveness analysis, has been considered as a necessary tool for an evidence-based economic evaluation to determine which drug or treatment is the most cost-effective. The study of cost-effectiveness is important for selecting a treatment method with lower costs and higher effectiveness. Therefore, by proposing more cost-effective treatment methods and emphasizing on their application for patients, health system resources can be maintained. Besides, the present finding can provide new evidence regarding the cost-effectiveness of treatments and help physicians and clinicians to select appropriate interventions. The results of this study can also help health policymakers, planners, and insurance organizations in allocating resources and making reimbursement decisions.
4.1. Limitations
Because of some limitations in database access and language restrictions, only the English literature was reviewed. The literature on this subject is very limited, and studies have been conducted in only nine countries, mostly high-income ones. Nonetheless, different countries have different healthcare, medical insurance, and reimbursement systems, as well as willingness-to-pay thresholds and gross domestic product. Therefore, there are certain limitations in extrapolating the data under review, and further studies are required in different countries, especially low-income ones, to evaluate the cost-effectiveness of EVAR in the treatment of patients with ruptured AAAs.
5. Conclusions
The results of the present study showed an improvement in the quality of life and a reduction in mortality and length of hospital stay among patients with ruptured AAAs undergoing EVAR compared to those undergoing OSR. The cost-effectiveness of EVAR has increased over time due to the development of high-quality medical equipment and the gradual improvement of physician experience and skills. Previous studies have been mostly performed in high-income countries, while the specific conditions of each country and the characteristics of each healthcare system should be considered for generalizing the results.