1. Background
Anatomical variations in vascular structures are frequently encountered. In the cerebellopontine region, anatomical variations, especially variations in the anterior inferior cerebellar artery (AICA) in relation to cranial nerves passing through the internal acoustic canal (IAC), are common (1). Magnetic resonance imaging (MRI) provides detailed information for the evaluation of the cerebellopontine region and the IAC anatomy (2). With recent advances in MRI technologies, small voxel sizes, acquired at a high magnetic field strength, have allowed for the optimal evaluation of anatomical features.
It is important to investigate and understand anatomical variations, especially before surgical procedures. T2-weighted (T2W) MRI with thin sections can successfully demonstrate the relationship between cranial nerves and vascular structures in patients with signs of neurovascular compression (2, 3). Previous studies have mostly examined the clinical symptoms of vascular loops of the AICA, but did not report any significant relationship (4-6). For example, Marques et al. evaluated the IAC morphology using computed tomography (CT) scan (7). However, there is no study in the literature on the relationship between the anatomical features of the IAC and AICA vascular loops.
2. Objectives
This study aimed to investigate the relationship between the IAC size and shape and the AICA variations in patients without pathological IAC lesions and to determine the distribution of anatomical variations and measurements by age and gender. It can be helpful for head and neck surgeons to understand the IAC anatomical features and relations with AICA loops before surgeries, such as IAC bone dissection for tumor removal. Such knowledge can be also useful for radiologists and anatomists to describe the anatomical features properly.
3. Patients and Methods
3.1. Patients
In the present study, the images of patients, who underwent contrast-enhanced temporal bone imaging using a 3-tesla MRI scanner between July 1, 2019 and December 31, 2019, were evaluated retrospectively. Patients older than 18 years were included in this study. The exclusion criteria were images of poor diagnostic quality due to technical or patient-related reasons (e.g., motion artifacts and metal-induced artifacts) and the presence of a tumor that could disrupt the IAC.
The contrast-enhanced temporal bone MRI images of 253 patients who met the criteria were acquired in this study. Four patients were excluded from the study, because they had a tumor associated with the cerebellopontine corner or IAC; the data of 249 patients were finally evaluated (Figure 1). Before the onset of the study, the local ethics committee approval and institutional permission were obtained. Informed consent was not required by the ethics committee due to the retrospective design of the study.
3.2. MRI Examination
Images were acquired using a Siemens Magnetom SkyraTM 3T MRI device (Siemens AG, Munich, Germany). After injecting 15 cc of an intravenous contrast agent (gadobutrol), fat-saturated T1W images were obtained in the axial plane according to the temporal bone imaging protocols with the following parameters: repetition time (TR), 8.4 ms; echo time (TE), 3.69 ms; number of excitations (NEX), 1; section thickness, 1 mm; matrix size, 288 × 264; and field of view, 180 × 180 mm. Also, to acquire T2W sampling perfection with application-optimized contrasts using different flip angle evolution (SPACE) images, the parameters were as follows: TR, 1200 ms; TE, 154 ms; NEX, 2; flip angle, 120°; echo train length, 69; section thickness, 0,6 mm; matrix size, 320 × 300; and field of view, 160 × 160 mm.
3.3. Imaging Analysis
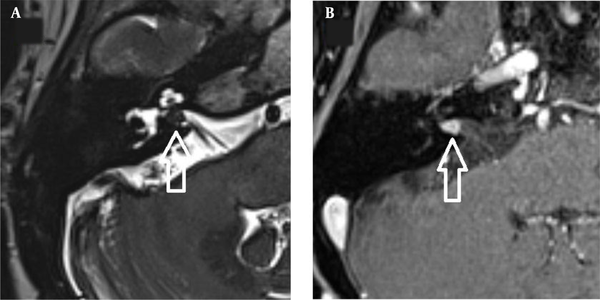
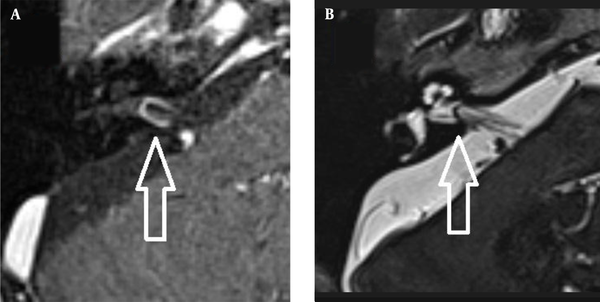
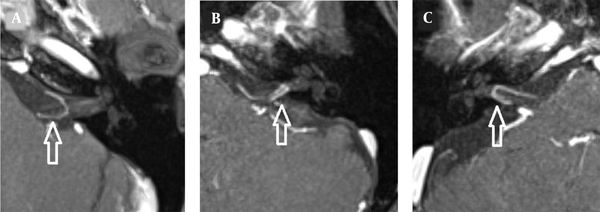
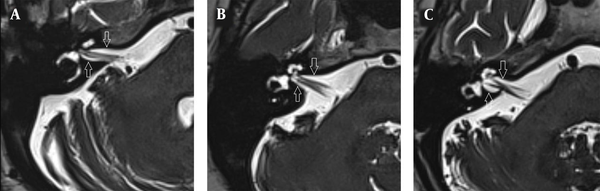
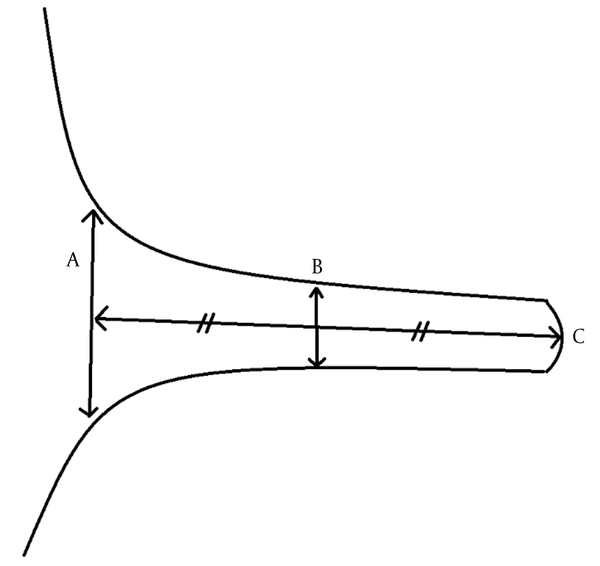
The images were evaluated by two radiologists (with six and two years of experience, respectively) based on consensus. The IAC was evaluated in terms of the presence of vascular loops using contrast-enhanced axial images on both sides. The contrast-enhanced T1W images provided a better contrast of the AICA to background and cranial nerves compared to T2W images (Figure 2). Patients with vascular loops were categorized according to the Chavda classification (3). According to this classification, a type 1 loop borders the internal auditory meatus, but does not enter the canal; a type 2 loop extends to the medial half of the canal; and a type 3 loop extends to the lateral half of the canal (Figure 3). The evaluation of the canal shape and dimensions was based on axial T2W SPACE images. The canals were also classified as cylindrical, funnel-shaped, and bud-shaped (Figure 4). The IAC measurements were performed in millimeters (mm) at 3× magnification (Figure 5).
3.4. Statistical Analysis
After the demographic data, collected from the patients’ hospital records, and the imaging findings were registered on a computer, statistical analysis was performed in IBM SPSS Version 20.0 (IBM Corp., Armonk, NY, USA). Continuous data with a normal distribution are expressed as mean ± standard deviation (minimum/maximum) and categorical data as frequency and percentage. A correlation analysis was performed using Pearson’s correlation test for parametric data to determine the correlation of the IAC diameter with the patient’s age. The mean IAC diameters were compared between different groups (gender, age group, canal shape, and Chavda classification) using independent samples t-test. Besides, categorical data, such as canal shape and Chavda classification, were compared using chi-square test. P < 0.05 was considered statistically significant.
4. Results
Of 249 patients included in the study, 145 (58.2%) were female, and 104 (41.8%) were male. The mean age of the patients was 45.65 ± 13.07 years (range: 18 - 80 years); the mean age of women was 43.84 ± 12.73 years (range: 18 - 73 years); and the mean age of men was 48.16 ± 13.18 years (range: 18 - 80 years).
On the right side, no vascular loop was observed in 153 (61.4%) patients. However, type 1 vascular loops were found in 60 (24.1%) cases, type 2 loops in 24 (9.6%) cases, and type 3 loops in 12 (4.8%) cases. On the left side, no vascular loop was observed in 146 (58.6%) cases, while type 1 vascular loops were found in 65 (26.1%) cases, type 2 in 24 (9.6%) cases, and type 3 in 14 (5.6%) cases. There was no significant difference between the right and left ears in terms of the presence of a vascular loop (P = 0.915).
In 498 inner ears, the mean diameter of the IAC was 4.56 ± 1.03 mm (range: 1.9 - 8.59 mm); the mean canal length was 9.62 ± 1.59 mm (range: 5.25 - 15.56 mm); and the mean meatus diameter was 6.02 ± 1.86 mm (range: 2.82 - 13.54 mm). In men, the mean diameter of the IAC was 4.46 ± 1.03 mm, the mean canal length was 9.41 ± 1.62 mm, and the mean meatus diameter was 5.83 ± 1.84 mm, while in women, the mean corresponding values were 4.64 ± 1.00 mm, 9.77 ± 1.54 mm, and 6.15 ± 1.86 mm, respectively. All diameters of the inner ear were higher in women compared to men; however, only the mean IAC diameter was significantly higher (P = 0.011 for the IAC diameter, P = 0.059 for the meatus diameter, and P = 0.058 for the canal length).
On the right side, the mean IAC diameter was 4.50 ± 0.99 mm, the mean canal length was 9.39 ± 1.62 mm, and the mean meatus diameter was 5.82 ± 1.84 mm, while on the left side, the mean IAC diameter was 4.63 ± 1.03 mm, the mean canal length was 9.86 ± 1.52 mm, and the mean meatus diameter was 6.22 ± 1.86 mm. The canal length and meatus diameter were slightly higher on the left side compared to the right side (P = 0.001 and P = 0.018, respectively). Of all IACs, 230 (46.2%) were cylindrical, 169 (33.9%) were funnel-shaped, and 99 (19.9%) were bud-shaped. The funnel-shaped and bud-shaped IACs were associated with the presence of a vascular loop (χ2 = 12.688, P = 0.048) (Table 1).
| Canal shape | No loops | Chavda type 1 | Chavda type 2 | Chavda type 3 | Total |
|---|---|---|---|---|---|
| Cylindrical | 152 (30.5) | 54 (10.8) | 15 (3) | 9 (1.8) | 230 (46.2) |
| Funnel-shaped | 99 (19.9) | 42 (8.4) | 20 (4) | 8 (1.6) | 169 (33.9) |
| Bud-shaped | 48 (9.6) | 29 (5.8) | 13 (2.6) | 9 (1.8) | 99 (19.9) |
| Total | 299 (60) | 125 (25.1) | 48 (9.6) | 26 (5.2) | 498 (100) |
Relationship Between the Internal Acoustic Canal (IAC) Shape and the Presence of Anterior Inferior Cerebellar Artery (AICA) Vascular Loops a
In cases without vascular loops, the mean diameter of the IAC was 4.25 ± 0.096 mm, the mean canal length was 9.38 ± 1.59 mm, and the mean meatus diameter was 5.60 ± 1.73 mm, while the mean corresponding values in the group of vascular loops were 5.03 ± 0.91 mm, 9.99 ± 1.51 mm, and 6.65 ± 1.87 mm, respectively. All of the IAC measurements were significantly higher in the group of vascular loops (P < 0.0001 for all) (Table 2). The IAC diameter and canal length showed weak negative correlations with the patient’s age, while the meatus diameter had a moderate negative correlation with age (r = -0.479, P < 0.0001; r = -0.387, P < 0.0001; and r = -0,598, P < 0.0001, respectively). Finally, when the patients were divided into two age groups of ≥ 65 and < 65 years, no significant difference was found between them in terms of the presence of a vascular loop (χ2 = 1.214, P = 0.271).
| Variables | IAC diameter A | Canal length B | Meatus diameter C | Significance level of t-test between the groups |
|---|---|---|---|---|
| Male | 4.46 ± 1.03 | 9.41 ± 1.62 | 5.83 ± 1.84 | P = 0.011 A, P = 0.059 B, P = 0.058 C |
| Female | 4.64 ± 1.00 | 9.77 ± 1.54 | 6.15 ± 1.86 | |
| Right side | 4.50 ± 0.99 | 9.39 ± 1.62 | 5.82 ± 1.84 | P = 0.130 A, P = 0.001 B, P = 0.018 C |
| Left side | 4.63 ± 1.03 | 9.86 ± 1.52 | 6.22 ± 1.86 | |
| Any type of AICA loop | 5.03 ± 0.91 | 9.99 ± 1.51 | 6.65 ± 1.87 | P < 0.0001 |
| No AICA loops | 4.25 ± 0.09 | 9.38 ± 1.59 | 5.60 ± 1.73 | |
| All inner ears | 4.56 ± 1.03 | 9.62 ± 1.59 | 6.02 ± 1.86 |
5. Discussion
In the present study, in line with the literature, the most common type of IAC-associated AICA loop was Chavda type 1, in which the arterial loop is located outside the IAC on both sides. Type 1 vascular loops were followed by type 2 and type 3 loops (4, 6, 8, 9). According to the present results, all IAC measurements showed a negative correlation with the patient’s age. Similarly, in a CT study, Marques et al. (7) found that the dimensions of the IAC were significantly larger in the pediatric group compared to the adults. However, we found no significant difference between the elderly patients aged ≥ 65 years and others in terms of the presence of AICA vascular loops; this suggests that tortuosity and ectasia, which may occur in vascular structures with age, are not related to the presence of an AICA vascular loop.
In this study, the most common IAC shape was cylindrical (46.2%), followed by funnel (33.9%) and bud (19.9%) shapes. In this regard, Marques et al. measured the frequency of funnel-shaped, cylindrical, and bud-shaped IAC and found differences in the IAC shape compared to the literature, which could be attributed to racial differences and anatomical structures affected by genetic changes during embryogenesis (7). The current study is the first to investigate the IAC shape in a Turkish population. The mean diameter of the IAC was 4.56 ± 1.03 mm (range: 1.9 - 8.59 mm), the mean canal length was 9.62 ± 1.59 mm (range: 5.25 - 15.56 mm), and the mean meatus diameter was 6.02 ± 1.86 mm (range: 2.82 - 13.54 mm).
In previous studies, the IAC dimensions have been examined radiologically by CT scan. Marques et al. found that the mean anteroposterior diameter of the IAC was 4.82 mm, the mean canal length was 11.17 mm, and the mean meatus diameter was 7.53 mm (7). Differences in the IAC size may be related to genetic and racial differences (similar to the shape of the canal). Based on the comparison of the IAC shapes regarding the presence of AICA vascular loops, the rate of vascular loops was higher in the funnel-shaped and bud-shaped IACs compared to cylindrical canals. This finding suggests that an arterial vascular loop may be related to the canal shape; however, there is no similar study in the literature.
In the present study, we focused on the anatomical features of the AICA and IAC rather than clinical symptoms related to AICA loop variations. So far, no significant relationship has been reported between arterial vascular loops and clinical symptoms, such as tinnitus or vertigo in previous studies (4-6). On the other hand, evaluating the IAC anatomy and variations before a surgical procedure, especially for tumors, not only facilitates tumor resection, but also preserves the labyrinth (10). Therefore, the IAC and AICA variations should be evaluated although they cause no clinical symptoms.
In previous studies, 3D T2W and constructive interference in steady-state (CISS) images were acquired to evaluate the AICA anatomy (9, 11). Leal et al. prospectively studied trigeminal nerve vascular compression with a combination of 3D T2W, contrast-enhanced T1W, and time of flight (TOF) sequences and reported vascular compression with high inter-observer reliability. On contrast-enhanced T1W images, the arteries showed good visualization due to higher signal intensity (12). Also, in the present study, the AICA was more visible in contrast-enhanced images compared to T2W images.
Contrast-enhanced T1W imaging is recognized as the standard protocol, along with 3D T2W and TOF sequences, especially for neurovascular compression detection (13). Accordingly, the present study aimed to improve the visualization of vascular structures using intravenous contrast-enhanced images in temporal bone MRI examinations and to detect tumors that can cause anatomical impairments. On the other hand, the borders of the IAC are better visualized on T2W images, which is a disadvantage of T1W imaging. Therefore, the presence of vascular loops was only evaluated on T1W images in this study, while the canal shape and dimensions were evaluated on T2W images. Besides, a slice thickness of 0.6 mm, provided by the 3T MRI machine, facilitated a better anatomical evaluation by T2W images as compared to previous studies using slice thicknesses of 0.7 to 0.8 mm (9, 11, 14).
The limitations of this study include its single-center design that limited the generalizability of our findings. Besides, due to the retrospective design of the study, the clinical symptoms could not be evaluated, and no imaging follow-up could be performed. Finally, the IAC measurements and shape evaluations were performed by two radiologists based on consensus; therefore, interobserver agreement could not be investigated.
In conclusion, knowledge of anatomical variations is important in surgical procedures. In previous studies, the AICA variations were evaluated clinically and symptomatically, while their relations with the anatomical features of IAC were not compared. The current study revealed a relationship between the size and shape of the IAC and the AICA loop variations. The present results showed that the AICA loop variations were closely related to the IAC shape and diameter.