1. Background
Knee osteoarthritis is a common cause of referral to clinics and one of the debilitating diseases with a good prognosis if treated surgically. However, surgery is not the method of choice for all patients with knee osteoarthritis because of surgical complications. Besides, no patient with osteoarthritis tends to undergo surgery (1). Therefore, early diagnosis and maintenance interventions are of great priority in these patients (2, 3).
Cartilage injury is the primary characteristic of osteoarthritis. As the tissue cartilage lacks blood vessels and neurons, the pain mechanism is complex in these patients, affecting the non-cartilage structures of the joint, including the synovium, bone, and soft tissue. Inflammation of the joint, change in cartilage, and bone turnover in the osteoarthritis process represent the potential important role of molecular mediators in the pain mechanism of osteoarthritis. The mechanism of pain in osteoarthritis constitutes the activation and distribution of local anti-inflammatory mediators, such as prostaglandins and cytokinases, and is accompanied by tissue injury, which is affected by proteases (4).
Biological lasers were first introduced 20 years ago (5). Because of its potential applications in biomedicine and biological research, this method has recently received particular attention (6-10). Biolaser or biological laser is a new type of laser, embedded or placed in part of a biological hole or environment (10). In the rehabilitation and physiotherapy of these patients, biolaser radiation with wavelengths of 600 - 980 nm with a low/medium energy level, such as gallium arsenide (GaAs) or helium neon (HeNe), is used. The application of this method appears to be successful in the management of chronic or acute musculoskeletal pain.
In the last 50 years, laser therapy has been used at low red light wavelengths in in vitro, in vivo and human studies (infrared radiation at wavelengths of 660 - 900 nm). Biolaser is specifically used for the management of degenerative knee osteoarthritis. The efficacy of this method in cartilage formation (11), cartilage erosion (12), production of super oxide dismutase enzymes (12), stress protein levels (13), and chondrocyte proliferation (13) has been assessed in previous research. In these studies, osteoarthritis was induced by the injection of chemical agents into the capsular segment of mouse and rabbit knee joints. The biolaser method decreases the joint inflammation process (11), joint cartilage reproduction (12), and stress protein levels and improves the repair of cartilage degeneration (13); it also increases the number of cartilage cells and the thickness of joint cartilage (14). In a previous meta-analysis, the biolaser method could significantly improve pain, using a 2 – 4 week therapeutic regimen at an optimal dose (15).
Currently, the biolaser method is used for the management of joint, neural, and soft tissue disorders. Considering the less invasive nature of this method, the reduced need for non-steroidal anti-inflammatory drugs (NSAIDs) and other pain killers, and improvement of the physical function of the knee in patients with osteoarthritis, researchers are paying special attention to biolasers. However, there are discrepancies regarding the application of low-level biolasers in alleviating pain in patients with knee osteoarthritis. The present study, for the first time, aimed to evaluate the clinical effectiveness of ultrasound-guided biolasers versus ozone therapy in alleviating chronic pain in knee osteoarthritis during a three-month follow-up in an Iranian population.
2. Objectives
For the first time, this study aimed to evaluate the clinical effectiveness of ultrasound-guided biolaser versus ozone therapy in reducing chronic pain in knee osteoarthritis during a three-month follow-up in an Iranian population.
3. Patients and Methods
3.1. Study Population
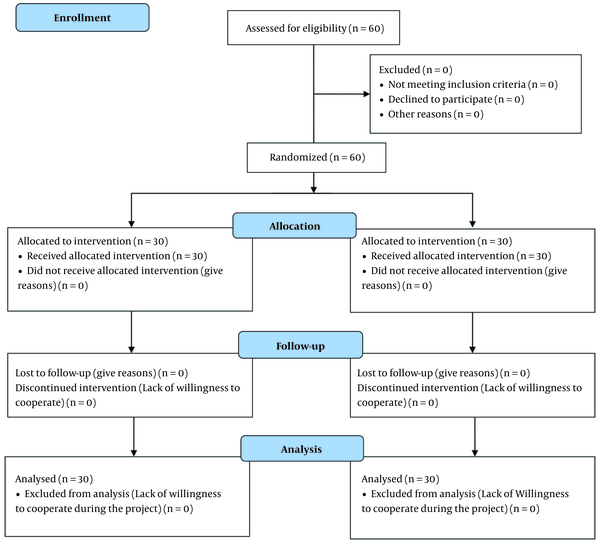
This single-blind randomized clinical trial was conducted on patients with knee osteoarthritis, referred to the pain clinics of Shohadaye-Tajrish and Akhtar hospitals (Tehran, Iran) in 2017. The inclusion criteria were consent to participate in the study, age range of 40 years or above, pain due to knee osteoarthritis for more than three months, and radiological findings of osteoarthritis according to the American College of Rheumatology (ACR) (16). On the other hand, the exclusion criteria were as follows: A history of knee surgery; deformity or contracture of the lower limbs; neuromuscular disorders of the lower limbs; acute lumbar pathologies; recent intraarticular steroid injections in the past two months; history of inflammatory rheumatoid arthritis, infection, or diabetes; pregnancy; breastfeeding; body mass index (BMI) > 35 kg/m2; being a candidate for knee surgery; genu varum or genu valgus > 5°, confirmed by 3D images joint-view; radicular pain of the knee; use of anticoagulants; posttraumatic arthrosis; ozone therapy in the past 12 months; systemic or psychiatric disorders; and severe osteoarthritis (grade > III) (Figure 1).
Sixty participants were randomly assigned to ultrasound-guided biolaser and ozone therapy groups (n = 30 per group), according to a random number table (with a series of digits from 0 to 9, arranged randomly in rows and columns).
3.2. Intervention
The patients assigned to the ozone therapy group received 10 cc of ozone (30 μg/mL), 5 mL of lidocaine 1%, placebo biolaser, and physical therapy (corrective exercise). The patients assigned to the biolaser group received 10 mL of normal saline, 5 mL of lidocaine 1%, and biolaser radiation plus physical therapy (corrective exercise). To perform the procedure, the patients were placed in the supine position, and the injection site landmarks were marked on the lateral side of the knee by flexing the knee by 30 - 45 degrees. The injection site was then disinfected with povidone-iodine. Using a 27-gauge needle, 2 mL of 2% lidocaine solution was injected, and the skin and joint surface were anesthetized. After aspiration and ensuring the correct placement of the needle, intraarticular ozone injection was performed under ultrasound guidance. Biolaser radiation was also carried out under ultrasound guidance.
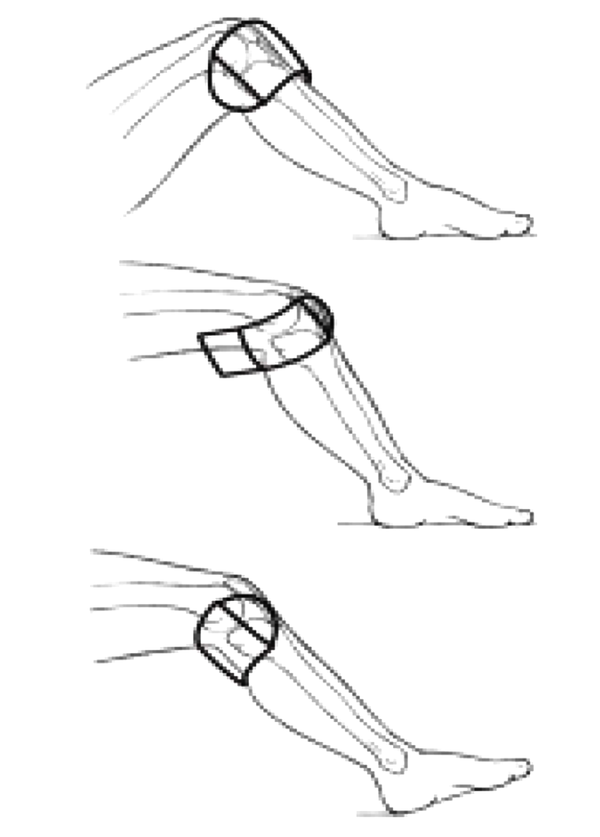
Ozone injection was performed weekly for three weeks; the final dose was injected one month after the third dose. The participants received biolaser and placebo therapies three times a week (every other day, a total of 12 sessions). The patients were blinded to the intervention. Next, the injection site was disinfected, and for biolaser radiation (Prob 890 MJ, Thor Company, UK), 30 mV per point was applied. For red and infrared superluminescent diodes (SLD), the knees were positioned on the lateral, medial, and popliteal sides. The therapeutic regimen involved three steps (Figure 2) (17) :
(1) Red light radiation (660 nm) using a flexible gaala laser diode 180 sld system (750 mW).
(2) Infrared radiation (840 nm) using a flexible gaala laser diode 180 sld system (1500 mW).
(3) Using an infrared laser probe (830 nm) with a flexible gaala laser with a focused laser source on the basic pathology (75 - 200 mW).
For complete management of the knee circumference, each SLD was placed in three lateral, medial, and popliteal positions. To penetrate into the patellar and posterior patellar spaces, the knee was flexed to 90° (Figure 2) (17). In each session, radiation was performed on three points (lateral, upper, and lower points of the patella, with a maximum patellar edge of 3 cm). At each point, 8 J of energy (total energy, 32 J) was radiated with a laser probe. In the placebo laser group, radiation was performed using a non-active probe. The participants did not take any steroidal, antidepressant, or sedative medications.
Five variables, including pain, symptoms, daily activities, athletic and recreational activities, and knee-related quality of life, were measured based on the Knee injury and Osteoarthritis Outcome Score (KOOS) (18), which was completed by the patients before and three months after injecting the final dose. The pain severity was assessed using a visual analog scale (VAS), with scores ranging from 0 to 10. The participants were asked to score their pain severity before the intervention, one month after the intervention, and three months after the intervention. Sensitivity to pressure was measured by the Ritchie Articular Index (0 = “No pain”; 1 = “Patient complained of pain”; 2 = “Patient complained of pain and winced”; 3 = “Patient complained of pain, winced, and withdrew the joint”) (19). Additionally, the knee circumference was measured by a physician. If any complication occurred during the study, it was recorded.
3.3. Statistical Analysis
Data were collected from the patients’ medical records; the collected data from the follow-ups, interviews, and visits were recorded in datasheets. All data were entered into SPSS version 26 (IBM Corp., released in 2019., IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, USA). After examining the normal distribution of quantitative data by Kolmogorov-Smirnov test, quantitative variables were compared between the two groups using t-test, Mann-Whitney test, paired t-test, and repeated measures ANOVA. Categorical variables were also analyzed using chi-square test. The significant level was considered to be less than 0.05.
4. Results
The demographic characteristics of the ozone therapy and biolaser radiation groups are presented in Table 1.
Comparison of the Demographic Information of the Participants Between the Ozone Therapy and Biolaser Radiation Groups
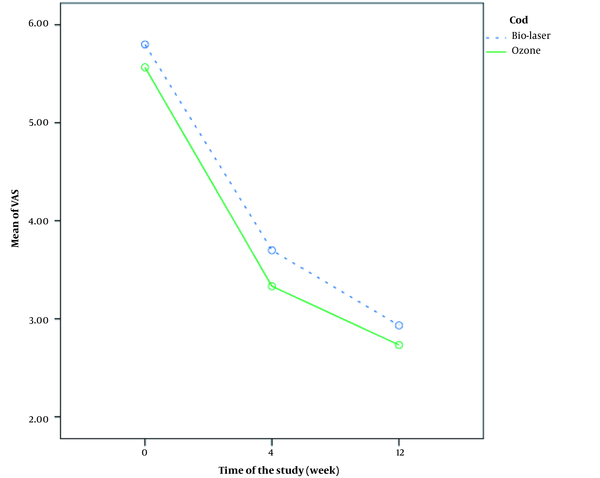
Comparison of pain severity changes based on the VAS score before, four weeks, and 12 weeks after treatment between the two groups is shown in Figure 3. Changes in pain severity were significantly different between the two groups, which suggests significant pain improvement in the biolaser group (P = 0.018). Comparison of pain severity at different intervals is presented in Table 2. There was a significant difference between the two groups four weeks after the onset of treatment (P = 0.005).
| Variables | Biolaser radiation(No = 30) | Ozone therapy(No = 30) | P-value |
|---|---|---|---|
| VAS score before | 5.7 ± 0.6 | 5.7 ± 1.0 | 0.262 |
| VAS score after four weeks | 3.5 ± 0.4 | 3.3 ± 0.5 | 0.005 |
| VAS score after 12 weeks | 2.6 ± 0.2 | 2.7 ± 0.6 | 0.160 |
| P-value (4 weeks vs. 12 weeks) | < 0.0001 | < 0.0001 |
Comparison of Pain Severity Between the Ozone Therapy and Biolaser Radiation Groups at Different Intervals
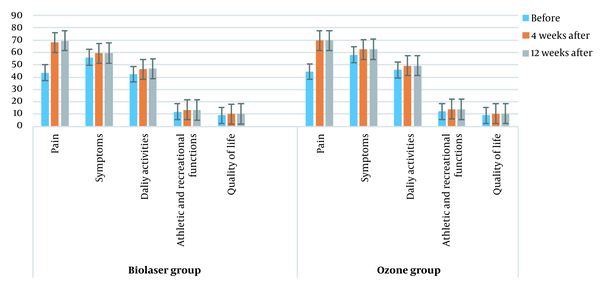
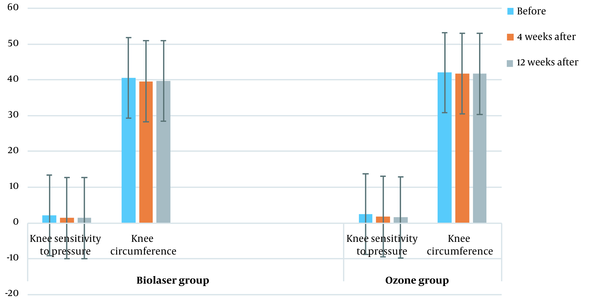
Comparison of KOOS score changes between the two groups in different intervals (before treatment, four weeks after treatment, and 12 weeks after treatment) is shown in Figure 4 (P > 0.05). Figure 5 presents a comparison of the knee pressure sensitivity between the two groups before treatment, four weeks after treatment, and 12 weeks after treatment (P < 0.05), as well as the knee circumference before treatment, four weeks after treatment, and 12 weeks after treatment (P < 0.05). Moreover, comparison of KOOS scores between the two groups at four and 12 weeks post-treatment is shown in Table 3.
| Variables | Biolaser radiation (No = 30) | Ozone therapy (NO = 30) | P-value |
|---|---|---|---|
| KOOS pain after four weeks | 68.1 ± 10.9 | 69.7 ± 11.8 | 0.339 |
| KOOS pain after 12 weeks | 69.5 ± 10.3 | 69.6 ± 11.2 | 0.835 |
| P-value | 0.609 | 0.977 | |
| KOOS symptoms after four weeks | 59.3 ± 5.2 | 62.5 ± 4.4 | 0.015 |
| KOOS symptoms after 12 weeks | 59.5 ± 4.7 | 62.6 ± 4.4 | 0.011 |
| P-value | 0.150 | 0.159 | |
| KOOS daily activities after four weeks | 46.4 ± 9.7 | 49.2 ± 7.9 | 0.164 |
| KOOS daily activities after 12 weeks | 46.9 ± 9.4 | 49.3 ± 7.9 | 0.187 |
| P-value | 0.183 | 0.161 | |
| KOOS athletic and recreational activities after four weeks | 13.4 ± 1.7 | 13.9 ± 1.8 | 0.349 |
| KOOS athletic and recreational activities after 12 weeks | 13.3 ± 1.6 | 13.9 ± 1.8 | 0.210 |
| P-value | 0.876 | 0.161 | |
| KOOS quality of life after four weeks | 10.2 ± 1.4 | 10.3 ± 1.4 | 0.516 |
| KOOS quality of life after 12 weeks | 10.3 ± 1.3 | 10.4 ± 1.5 | 0.586 |
| P-value | 0.293 | 0.161 |
Comparison of KOOS Scores Between the Ozone Therapy and Biolaser Radiation Groups
Table 4 presents a comparison of the knee sensitivity to pressure, as well as the knee circumference in different intervals between the groups.
| Variables | Biolaser radiation (No = 30) | Ozone therapy (No = 30) | P-value |
|---|---|---|---|
| Knee sensitivity to pressure (before treatment) | 2.5 ± 0.6 | 2.2 ± 0.9 | 0.043 |
| Knee sensitivity to pressure (four weeks after treatment) | 1.8 ± 0.6 | 1.4 ± 0.5 | 0.010 |
| P-value | 0.0001 | 0.0001 | |
| Knee sensitivity to pressure (four weeks after treatment) | 1.8 ± 0.6 | 1.4 ± 0.5 | 0.010 |
| Knee sensitivity to pressure (12 weeks after treatment) | 1.3 ± 0.5 | 1.6 ± 0.4 | 0.037 |
| P-value | 0.022 | 0.022 | |
| Knee circumference (before treatment) | 42.0 ± 3.2 | 40.6 ± 4.2 | 0.325 |
| Knee circumference (four weeks after treatment) | 41.6 ± 3.0 | 39.6 ± 4.0 | 0.037 |
| P-value | 0.010 | 0.002 | |
| Knee circumference (four weeks after treatment) | 41.6 ± 3.0 | 39.6 ± 4.0 | 0.032 |
| Knee circumference (12 weeks after treatment) | 35.6 ± 3.0 | 39.7 ± 3.9 | 0.032 |
| P-value | 0.0001 | 0.161 |
Comparison of the Knee Sensitivity to Pressure and the Knee Circumference Between the Ozone Therapy and Biolaser Radiation Groups
No complication was attributed to the study procedures during the study.
5. Discussion
In the current study, the pain severity significantly reduced in both groups in different intervals. The knee function and knee-related quality of life showed significant improvements after four weeks of treatment; however, no significant improvement was observed 12 weeks after treatment. The knee function and knee-related quality of life were better in the biolaser radiation group compared to the ozone therapy group; nevertheless, no significant difference was found between the two groups. In the present study, knee sensitivity to pressure and knee circumference significantly reduced in the biolaser radiation group. These findings are consistent with several previous reports (15, 20, 21).
Low-level laser therapy may intensify the biological effects by increasing adenosine triphosphate (ATP) production in the cell mitochondria; these biological effects contribute to the regulation of transcription factors, as well as anti-apoptosis and pro-survival genes (22, 23). Biological events in the tissue induce protein synthesis, increase cellular migration and proliferation, and modulate regulatory cytokines, growth factors, inflammatory mediators, tissue oxygenation, and remodeling (24-26). Therefore, one of the most important clinical goals of low-level laser therapy is the management of tissue inflammation and degeneration.
Since osteoarthritis has a complex pathogenesis, red and infrared light radiation was performed at wavelengths of 660, 840, and 830 nm; these wavelengths were used for activation of different cellular pathways in several basic and clinical studies (27-29). According to several reports, laser radiation at a wavelength of 660 nm leads to the electric stimulation of most biomolecules, such as cytochrome C oxidase (30-32). Previous reports show that laser radiation at a wavelength of 905 nm induces microthermal gradients and selective photothermolysis of mitochondria. Therefore, laser radiation at wavelengths of 660, 840, and 830 nm can be tolerated by cellular chromophores (22, 23).
In some studies, it was shown that the wavelengths used in the biolaser affect mitochondrial chromophores through photochemical reactions (23). Accordingly, a combination of 660, 840, and 830 nm wavelengths increases and strengthens the biological effects with high probability. Lievens and van der Veen. showed that a combination of laser wavelengths increases fibroblastic proliferation in cell injuries and produces better clinical results for the improvement of an injured connective tissue (33). Another study indicated the probable synergic effects of laser therapy at two different continuous wave and pulsed wavelengths in the management of peripheral nerve injuries (34).
Biomolecular pathways are the target of all biological responses; consequently, wavelength is one of the important characteristics of laser therapy. Considering the photochemical reactions, red light radiation (660 nm) may target the mitochondria of cytochrome C oxidase, and infrared radiation (840 and 830 nm) initiates photochemical reactions in the lipid membranes of mitochondria. Accordingly, a combination of two or three wavelengths may improve the therapeutic effects of low-level laser therapy. The combination of photochemical and photophysical reactions in low-level laser therapy can also yield positive therapeutic results.
In conclusion, based on the present results, biolaser therapy under ultrasound guidance is a safe, non-invasive, and effective method that can improve chronic pain in knee osteoarthritis during a three-month follow-up. Therefore, application of the therapeutic biolaser protocol of the present study can be recommended to chronic pain specialists, orthopedic surgeons, and rheumatologists.