1. Background
Hepatocellular carcinoma (HCC) is characterized by high morbidity and mortality rates around the world, ranking the sixth most common malignant tumor and the second cause of mortality due to malignant tumors (1, 2). Sorafenib, as the first oral anti-angiogenic multikinase inhibitor, which has been approved for the treatment of advanced HCC, can block the root abundant factor (RAF) signaling pathway and inhibit the expression of vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), and v-kit Hardy-Zuckerman 4 feline sarcoma viral oncogene homolog (KIT) (3).
In recent years, new promising therapeutic strategies for HCC, such as immune checkpoint inhibitors (ICIs), have been investigated. Nivolumab was the first ICI, approved by the Food and Drug Administration (FDA) for the treatment of progressing advanced HCC in patients receiving sorafenib (4). Since then, preclinical and clinical trials have been carried out on several monoclonal antibodies, which target immunosuppressive molecules, inhibiting T-cell activation and promoting the depletion of T cells (5), such as programmed cell death protein 1 (PD-1), for the treatment of HCC (4, 6, 7); however, the effect of single therapy with the anti-PD-1 antibody is not ideal (8). Since tumors use various mechanisms to evade the immune surveillance, treatment of HCC has been gradually combined with immunotherapy.
The combination of ICIs with anti-angiogenic therapy is a research hotspot in the treatment of HCC. The latest National Comprehensive Cancer Network (NCCN) clinical practice guidelines for hepatobiliary cancers clearly state that atezolizumab, combined with bevacizumab, is the first-line therapy for HCC, marking the beginning of an immunological era of first-line therapy for advanced HCC (9). Anti-angiogenesis therapy has been reported to enhance the anti-PD-1/PD-L1 activity by reducing VEGF-mediated immunosuppression and promoting intra-tumor T cell infiltration (10, 11). Meanwhile, a previous study indicated that 20.3% of HCC patients developed a progressive disease (PD) after receiving atezolizumab combined with bevacizumab (12). Besides, treatment becomes more challenging for HCC patients, who are refractory to anti-angiogenic therapy combined with ICIs.
Transarterial chemoembolization (TACE) integrating targeted chemotherapy can cause arterial embolization and ischemic necrosis, which improved the survival of patients with unresectable HCC (13, 14). It has been reported that TACE can stimulate the body to produce anti-tumor immunity (15). In previous research, TACE combined with ICIs/anti-angiogenic therapy has been reported to be effective in the treatment of unresectable HCC (16-18). Therefore, TACE may exhibit good efficacy for HCC patients with PD after anti-angiogenic therapy combined with ICIs. However, there are no studies on the efficacy or safety of TACE for these refractory patients. Accordingly, in this retrospective study, we aimed to explore the treatment efficacy and safety of TACE for refractory HCC patients after anti-angiogenic therapy combined with ICIs.
2. Objectives
In this study, we aimed to explore the treatment efficacy and safety of TACE for refractory HCC patients after anti-angiogenic therapy combined with ICIs.
3. Patients and Methods
3.1. Patients
The Institutional Review Committee of Wuhan Third Hospital approved this study. All the patients signed an informed consent form. From July 2019 to October 2022, a total of 34 consecutive patients developed PR after anti-angiogenic therapy combined with ICIs and underwent TACE. According to the immune response evaluation criteria in solid tumors (iRECIST) (19), two consecutive computed tomography (CT) or magnetic resonance imaging (MRI) assessments were performed on all the patients following anti-angiogenic therapy combined with ICIs, the results of which demonstrated an increased tumor diameter, new lesions, or distant metastases.
The inclusion criteria were as follows: (1) HCC patients diagnosed according to the European Association for the Study of the Liver (EASL) criteria and age > 18 years [1]; (2) patients who received TACE due to PD after anti-angiogenic therapy combined with ICIs; (3) Child-Pugh class A or B; and (4) an Eastern Cooperative Oncology Group (ECOG) score < 2. On the other hand, the exclusion criteria were as follows: (1) receiving other treatments, such as ablation therapy or radiotherapy; (2) having portal vein thrombosis; and (3) loss to follow-up (Figure 1).
3.2. TACE Procedure
A 5-F catheter (Cook Medical, Bloomington, Indiana, USA) or a 2.7-F microcatheter (PROGREAT®, Terumo, Tokyo, Japan) was placed into a hepatic tumor-supplying artery. First, an emulsion of 2-20 mL lipiodol and 20-60 mg epirubicin was injected into tumor arteries, and then, gelatine sponge particles (350 - 710 μm; Alicon, Hangzhou, China) were injected. Finally, an angiographic reexamination confirmed that the tumor vessels had been completely embolized.
3.3. Anti-angiogenic Therapy Combined with ICIs
According to the recommendations of the multidisciplinary tumor boards, the patients continued to receive anti-angiogenic therapy in combination with ICIs for two weeks after TACE, using the same regimen and dose as the pre-TACE stage. In this study, 16 patients received atezolizumab (1200 mg, every three weeks) and bevacizumab (15 mg/kg, every three weeks) before TACE and continued with this regimen following TACE. The other 18 patients received camrelizumab (200 mg, every three weeks) and apatinib (500 mg/day), with the same regimen as the pre-TACE stage.
3.4. Follow Up and Repeated TACE
All the patients evaluated in this study were followed-up until October 31, 2022. After the initial TACE, abdominal contrast-enhanced CT, MRI, and laboratory examinations were performed every six weeks. The results of follow-up CT or MRI were compared with the preoperative imaging findings, and the objective response rate (ORR) and disease control rate (DCR) of liver tumors were determined based on the modified RECIST (20). Generally, ORR is defined as complete response (CR) or partial response (PR), and DCR is classified as CR, PR, or stable disease (SD). If the imaging results showed residual tumors, TACE was repeated.
3.5. Definition and Evaluation of Data
The progression-free survival (PFS) was defined as the interval between the initial TACE and disease progression. The Common Terminology Criteria for Adverse Events (CTCAE) version 5.0 were used to assess the adverse events (AEs).
3.6. Statistical Analysis
The PFS was plotted using the Kaplan-Meier method. The Cox proportional-hazards regression model was used to analyze the potential prognostic factors affecting PFS. A two-tailed P-value less than 0.05 indicated a statistically significant difference. SPSS Version 24.0 (IBM Corp. Released 2016. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp.) was used for statistical analyses.
4. Results
4.1. Study Population and Patient Characteristics
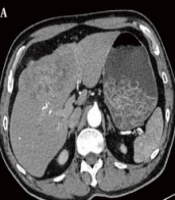
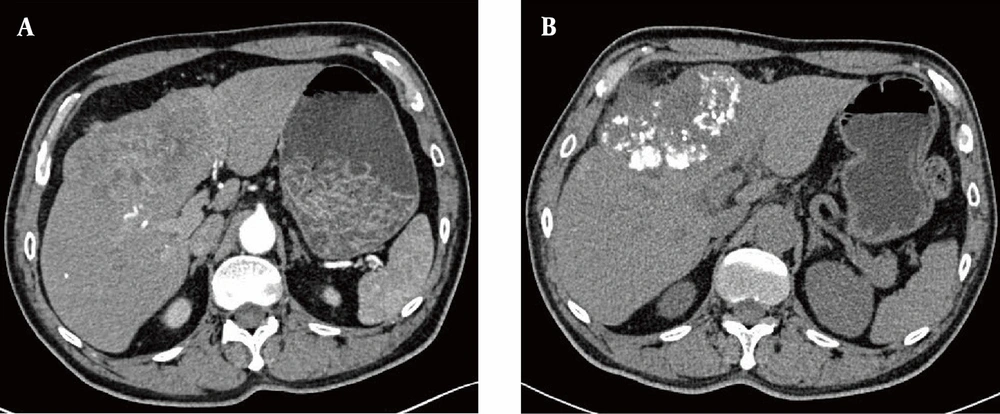
A total of 34 patients, including 26 males and 8 females (average age, 54.24 ± 11.91 years), were included in this study from July 2019 to October 2022. Overall, 23 patients underwent repeated TACE, with a mean time of 3.53 ± 3.60 times (range: 1 - 19 times). The detailed characteristics of 34 patients are presented in Table 1. The median follow-up period was 20 months. At the end of follow-up (October 31, 2022), 12 (35.3%) patients expired during the observation period. Figure 2 represents a 60-year-old male with HCC in the left inner lobe of the liver (S4), receiving TACE combined with anti-angiogenic therapy and ICIs. The follow-up CT at three months showed dense lipiodol deposition and CR of the tumors.
| Characteristics | Patients with UP after TACE | HR (95% CI) | P-value |
|---|---|---|---|
| Sex | |||
| Male | 26 (76.5) | 1 | |
| Female | 8 (23.5) | 0.900 (0.356, 2.273) | 0.823 |
| Age (y) | 54.2 ± 11.9 | 1.005 (0.972, 1.038) | 0.773 |
| ECOG | |||
| 1 | 20 (58.8) | 1 | |
| 0 | 14 (41.2) | 1.093 (0.500, 2.390) | 0.824 |
| BCLC stage | |||
| C | 27 (79.4) | 1 | |
| B | 7 (20.6) | 0.914 (0.344, 2.426) | 0.856 |
| Largest diameter of tumor (cm) | 8.1 ± 4.7 | 0.980 (0.897, 1.071) | 0.655 |
| Hepatitis | |||
| Hepatitis B | 28 (82.4) | 1 | |
| Others | 6 (17.6) | 1.041 (0.391, 2.766) | 0.936 |
| Alpha-fetoprotein level (ng/mL) | |||
| > 400 | 17 (50.0) | 1 | |
| ≤ 400 | 17 (50.0) | 0.903 (0.411, 1.984) | 0.799 |
| Child-Pugh score | |||
| B | 5 (14.7) | 1 | |
| A | 29 (85.3) | 1.408 (0.419, 4.733) | 0.580 |
| TACE sessions | 3.7 ± 3.4 | 1.036 (0.934, 1.150) | 0.500 |
| Tumor count | |||
| ≥3 | 29 (85.3) | 1 | |
| < 3 | 5 (14.7) | 1.133 (0.384, 3.341) | 0.821 |
| Ascites | |||
| Present | 4 (11.8) | 1 | |
| Absent | 30 (88.2) | 1.885 (0.439, 8.091) | 0.394 |
| Extrahepatic spread | |||
| Present | 11 (32.4) | 1 | |
| Absent | 23 (67.6) | 1.403 (0.587, 3.354) | 0.446 |
| Vascular invasion | |||
| Present | 15 (44.1) | 1 | |
| Absent | 19 (55.9) | 1.083 (0.500, 2.345) | 0.840 |
| Baseline laboratory test results | |||
| TB (μmol/L) | 16.8 ± 8.9 | 1.032 (0.992, 1.073) | 0.122 |
| Albumin (g/L) | 34.9 ± 3.8 | 1.023 (0.917, 1.141) | 0.687 |
| PT (s) | 14.1 ± 1.1 | 0.845 (0.578, 1.236) | 0.385 |
| AST (μmol/L) | 61.5 ± 56.2 | 1.007 (1.000, 1.013) | 0.048 |
| ALT (μmol/L) | 39.6 ± 20.3 | 1.001 (0.983, 1.020) | 0.887 |
| Creatinine (μmol/L) | 65.0 ± 18.8 | 0.995 (0.973, 1.017) | 0.637 |
| PLR | 189.7 ± 126.0 | 1.002 (0.999, 1.005) | 0.123 |
| NLR | 4.1 ± 4.2 | 1.051 (0.970, 1.138) | 0.226 |
Abbreviations: PFS, progression-free survival; HR, hazard ratio; UP, untreatable progression; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; BCLC, Barcelona Clinic Liver Cancer; TB, total bilirubin; PT, prothrombin time; AST, aspartate aminotransferase; ALT, alanine aminotransferase; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; TACE, transarterial chemoembolization.
a Values are expressed as No. (%) or mean ± standard deviation.
The computed tomography (CT) images before and after treatment. A, Axial CT scan after IV contrast medium injection in the arterial phase indicates hepatocellular carcinoma (HCC) located in the segment S4; B, Axial CT scan without IV contrast medium injection three months after transarterial chemoembolization (TACE), combined with anti-angiogenic therapy and immune checkpoint inhibitors (ICIs), indicates a reduced tumor volume and dense lipiodol deposition.
4.2. Efficacy Outcomes
The CT and MRI results at three months after the initial TACE showed 1 (2.9%) patient with CR, 8 (23.5%) patients with PR, 11 (32.4%) patients with SD, and 14 (41.2%) patients with PD. Accordingly, the ORR was 26.4%, and the DCR was 58.8%. Moreover, CT and MRI imaging at six months after the intervention showed 1 (2.9%) patient with CR, 4 (11.7%) patients with PR, 14 (41.2%) patients with SD, and 15 (44.1%) patients with PD; therefore, ORR was estimated at 14.6%, and DCR was estimated at 55.9%.
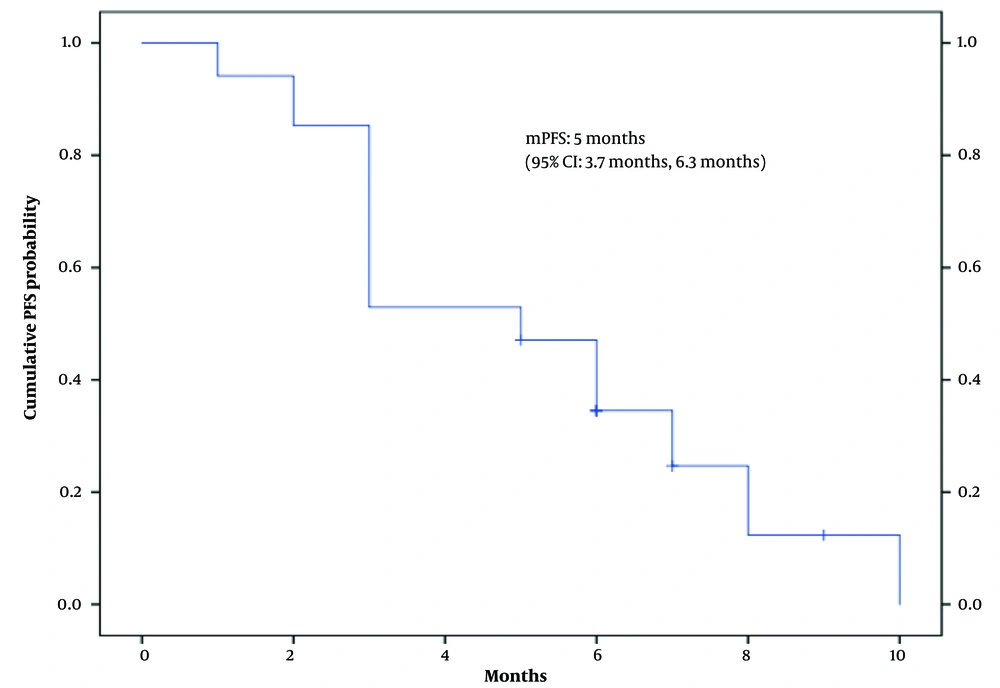
Additionally, the median follow-up period was 20 months (range: 2 - 30 months). Of 34 patients evaluated in this study, 27 (79.4%) showed disease progression. The median PFS of the patients was five months (95% CI: 3.7 - 6.3) (Figure 3). In the univariate analysis according to the Cox proportional-hazards model, the level of aspartate aminotransferase (AST) was significantly associated with PFS (P < 0.05) (Table 1).
4.3. Safety Outcomes
Within one week after TACE, 12 (35.3%) patients developed post-embolization syndrome, including fever (n = 8), abdominal pain (n = 9), and nausea and vomiting (n = 8). However, they showed improvements after symptomatic treatment. No severe TACE-related AEs occurred in any of the patients. During the follow-up, one or more types of AEs were observed in 10 (58.8%) patients after treatment with atezolizumab and bevacizumab (Table 2), and severe AEs (> grade III) did not occur in any of the patients. The most common AE caused by atezolizumab and bevacizumab was hypertension in 8 (47.1%) patients, followed by abnormal liver function (n = 6), asthenia (n = 5), rash (n = 2), and proteinuria (n = 1). Moreover, 12 (70.5%) patients developed one or more types of AEs after treatment with camrelizumab and apatinib (Table 2). The most common AE was reactive cutaneous capillary endothelial proliferation (RCCEP) (n = 9), followed by hand and foot skin reactions (n = 5), hypertension (n = 3), hypothyroidism (n = 2), diarrhea (n = 2), fatigue (n = 3), and rash (n = 2). No treatment-related mortality was reported.
| AEs | Atezolizumab plus bevacizumab (n = 16) | Camrelizumab plus apatinib (n = 18) | ||||||
|---|---|---|---|---|---|---|---|---|
| All AEs | CTCAE grade | All AEs | CTCAE grade | |||||
| 1 | 2 | 3 | 1 | 2 | 3 | |||
| Hypertension | 8 (50.0) | 5 (31.3) | 3 (18.7) | 0 (0) | 3 (16.7) | 1 (5.6) | 1 (5.6) | 1 (5.6) |
| Rash | 2 (12.5) | 1 (6.3) | 1 (6.3) | 0 (0) | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 (0) |
| Hepatic dysfunction | 6 (37.5) | 0 (0) | 4 (25.0) | 2 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Asthenia | 5 (31.3) | 3 (18.7) | 2 (12.5) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Proteinuria | 1 (6.3) | 0 (0) | 1 (6.3) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| RCCEP | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 9 (50.0) | 5 (27.8) | 3 (16.7) | 1 (5.6) |
| Hand and foot skin reactions | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 5 (27.8) | 2 (11.1) | 2 (11.1) | 1 (5.6) |
| Hypothyroidism | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 (0) |
| Diarrhea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 3 (16.7) | 2 (11.1) | 1 (5.6) | 0 (0) |
| Fatigue | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (11.1) | 1 (5.6) | 1 (5.6) | 0 (0) |
Abbreviations: CTCAE, Common Terminology Criteria for Adverse Events; RCCEP, reactive cutaneous capillary endothelial proliferation; AE, adverse events.
a Values are expressed as No. (%).
5. Discussion
The TACE has been reported to activate hypoxia responses and promote the release of proangiogenic factors, such as VEGF (21). Generally, tumors with low mutation rates and few antigens are less immunogenic, and therefore, less responsive to immunotherapy. According to a previous report, TACE can kill HCC cells, release tumor-associated antigens, and boost a tumor-specific CD8+ T cell response (15). Therefore, TACE, combined with anti-angiogenesis therapy and ICIs, can produce a strong tumor-specific immune response and inhibit tumor angiogenesis in the treatment of intermediate and advanced HCC; consequently, its clinical efficacy is favorable. Besides, Zheng et al. demonstrated the good efficacy and safety of TACE combined with sorafenib and ICIs for TACE-refractory HCC (16). Accordingly, we hypothesized that TACE could alter the tumor immune microenvironment and possibly improve the therapeutic effects in patients with a poor response to anti-angiogenic therapy plus ICIs.
In this study, ORR and DCR were estimated at 26.4% and 58.8% at three months after TACE, respectively, and ORR and DCR were 14.6% and 55.9% at six months after TACE, respectively; therefore, TACE combined with anti-angiogenesis therapy plus ICIs could result in favorable tumor control. Currently, anti-angiogenic therapy combined with ICIs is the first-line treatment for advanced HCC; however, some patients do not respond to this combination therapy. Therefore, further improvement of the efficacy of anti-angiogenic therapy combined with ICIs is an urgent problem in the treatment of HCC. In the present study, 34 HCC patients with tumor progression after combination therapy were retrospectively analyzed. The results showed that TACE combined with anti-angiogenic therapy and ICIs could benefit the patients, providing new insights for the treatment of patients with tumor progression after combination therapy.
In this study, 64.7% of the patients experienced AEs, but not more than grade III. Compared to nivolumab and pembrolizumab, the incidence of RCCEP is reportedly higher following camrelizumab treatment (22), and it has been reported in about 76.7% to 97.3% of other tumors (but not grade III RCCEP) (23). Similar to a study by Qin et al., RCCEP was the most common AE (24). However, grade III AEs or higher grades of AEs following camrelizumab treatment appeared to be rarer than other ICIs (17, 25), indicating the safety of anti-angiogenic therapy and ICIs for the treatment of HCC.
This retrospective study had several limitations. First, it was conducted in a single institution on a small sample size, without comparisons or controls, which limited the generalizability of the results. Second, the median overall survival was not available in this study, as most patients were still alive. Finally, this study did not make comparisons with other second-line treatments.
In conclusion, this study first reported TACE for HCC patients with tumor progression after anti-angiogenic therapy combined with ICIs. The results suggest that TACE may improve the tumor microenvironment and enhance the efficacy of anti-angiogenic therapy combined with ICIs for HCC. Overall, this study can be a reference for further prospective research to evaluate the efficacy of TACE against HCC in patients, who were not responsive to anti-angiogenic therapy in combination with ICIs.