1. Background
Dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) is a sensitive tool for detecting breast cancer, with reported sensitivities as high as 94 to 100% (1, 2). MRI has widely demonstrated diagnostic value in breast imaging by providing high-resolution morphological imaging along with information about contrast-enhanced characteristics (2-4). DCE-MRI accurately suggests the appropriateness of breast-conserving therapy (BCT) for patients whose MR images clearly demonstrate resectability of the tumor, and MRI is the only imaging modality capable of providing this information (5). If a breast cancer patient needs a mastectomy or neoadjuvant chemotherapy (NAC), a breast MRI can provide accurate information about the extent of the lesion, particularly when only suspicious findings are obtained from conventional mammography or when there is multicentric, nipple-areolar complex involvement and a mass size larger than 4 cm (5).
Conversely, reported DCE-MRI specificities are variable (37 to 99.7%), administering a gadolinium-based contrast agent for DCE-MRI is contraindicated for patients with renal insufficiency or a previous allergic reaction, and this process is time consuming (1, 2). Recent studies demonstrated the potential contribution of unenhanced imaging techniques to differentiate benign and malignant lesions, either alone or in combination with DCE-MRI (6-10). The non-enhanced T2 sequence is useful as an adjunct to contrast-enhanced sequences and offers the potential to improve the differential diagnoses of benign and malignant lesions (6, 7, 10-12). However, the significance and diagnostic impact of the non-enhanced T2 sequence alone are still being established clinically (8, 13). There are only a few reports regarding the usefulness of the pre-contrast T1 sequence.
The 5th Edition of American College of Radiology Breast Imaging Reporting and Data System was updated in 2013 to provide further clarification of image interpretation and standardize lesion terminology and reporting (14, 15). Several characteristic findings associated with post-biopsy change have been defined in breast cancer patients who have a diagnosis confirmed by a core needle biopsy. The associated findings include a pre-contrast high signal on T1 weighted images (WI), edema, skin thickening, and a post-biopsy hematoma (14). However, the associated findings are rarely evaluated on pre-contrast images.
We hypothesized that characteristic findings are present on pre-contrast T1WI and T2WI, including post-biopsy changes, and that these preoperative breast MRI findings can have a clinical impact on surgical decision-making. Knowledge of characteristic findings on pre-contrast T1WI and T2WI will enable more accurate decisions for treatment and management.
2. Objectives
The purpose of this study was to describe characteristic findings on preoperative pre-contrast MRI images in patients with recently diagnosed breast cancer confirmed by core needle biopsy and to evaluate the clinical impact of pre-contrast T1WI or T2WI.
3. Patients and Methods
3.1. Patient Population
This study was performed in accordance with the regulations of the institutional review board at our hospital, which approved this retrospective study and waived the requirement for informed patient consent. Of the 1,243 patients who underwent breast MRI between January 2010 and December 2012, 501 did not undergo a preoperative evaluation, 40 did not undergo a preoperative core needle biopsy, 16 did not undergo surgery at our hospital, and 296 were scanned with a different imaging setting. Therefore, a total of 390 patients were enrolled. The mean patient age was 51.7 ± 10.7 (range, 24 - 79) years. The mean interval from the core needle biopsy to breast MRI was 13.5 ± 8 days. The range of days between biopsy and MRI was 0 to 89 days.
3.2. Image Acquisition
MRI scans were acquired with the patient in the prone position in a 3-T scanner (Magnetom Verio; siemens medical solutions, Erlangen, Germany) equipped with a breast coil. MRI images were acquired with a Verio scanner using the following sequences: axial, turbo spin-echo T2WI sequence (TR/TE 4530/93 ms, flip angle 80°, 34 slices with FOV 320 mm, matrix 576 × 403, 1 NEX, 4 mm slice thickness, and acquisition time 2 minutes 28 seconds), pre- and post-contrast axial T1WI flash three-dimensional images, VIBE sequence (TR/TE 4.4/1.7, flip angle 10°, 1.2 mm slice thickness with no gap, acquisition time 60 seconds) obtained before and at 7, 67, 127, 187, 247, and 367 s after a bolus injection of 0.1 mmol/kg gadolinium-diethylenetriamine penta-acetic acid (Gd-DPTA).
3.3. Imaging and Clinical Analysis
Images and records were retrospectively evaluated by the consensus of two breast radiologists (B.K. and Y.A.) with 12 and 6 years experience in breast imaging, respectively. All imaging findings were classified with regard to the American college of radiology breast imaging reporting and data system (BI-RADS) MRI categories (14).
Approaches to treatment were based on the tumor node metastasis system (16). Mastectomy was considered for more extensive disease, such as multicentric disease, nipple-areolar complex involvement, or large cancers with a small breast on contrast-enhanced MRI (5).
Tumor size on contrast-enhanced MRI was determined as the greatest dimension of macroscopic disease across the three orthogonal dimensions. Nipple-areolar complex involvement as well as multifocal or multicentric lesions was evaluated on contrast-enhanced MRI.
We evaluated correlations between pre-contrast MRI features and DCE-MRI features, pre-contrast MRI features and clinical features, and the usefulness and clinical impact of pre-contrast MRI.
3.4. Histopathological Analysis
Histological records were reviewed for cancer type, size, and nipple-areolar complex involvement on surgical specimens.
3.5. Statistical Analysis
Data are presented as the mean ± standard deviation (range), or numbers (%). Statistics were performed using the chi-square test, Fisher’s exact test, and the Wilcoxon rank-sum test. All analyses were performed with SAS ver. 9.1 (SAS Institute, Cary, NC, USA). P values less than 0.05 were considered significant.
4. Results
4.1. Image Findings
Pre-contrast T1WI and T2WI findings, which were assumed to associate with post-core needle-biopsy changes, were noted in 27.9% of cases (n = 109/390). In addition, certain findings indicated various clinical conditions. Subareolar ductal pre-contrast hyperintensity on T1WI (9%, n = 35) indicated a subareolar ductal hemorrhage, whereas intra-lesional pre-contrast hyperintensity on T1WI (8.7%, n = 34) indicated an intra-lesional hemorrhage. Pre-contrast hyperintensity on T2WI (1.3%, n = 5) indicated hemorrhagic or cystic changes, while trabecular thickening on T2WI (9%, n = 35) indicated subcutaneous/peri-tumoral edema (Table 1).
| Variable | Mean ± Standard Deviation (Range) or No. (%) |
|---|---|
| Age (year) | 51.7 ± 10.7 (24 - 79) |
| Size (cm) | 2.7 ± 2.0 (0 - 12) |
| Intervala (day) | 13.5 ± 8.0 (0 - 89) |
| T1, Subareolar Ductal Hemorrhage | |
| Negative | 355 (91.03) |
| Positive | 35 (8.97) |
| T1, Intra-Lesional Hemorrhage | |
| Negative | 356 (91.28) |
| Positive | 34 (8.72) |
| T2, Hemorrhage or Cystic Change | |
| Negative | 385 (98.72) |
| Positive | 5 (1.28) |
| T2, Edema | |
| Negative | 355 (91.03) |
| Positive | 35 (8.97) |
| Operation Method | |
| Breast-conserving surgery | 268 (68.72) |
| Mastectomy | 122 (31.28) |
| Cancer Type | |
| Ductal carcinoma in situ | 62 (15.90) |
| Invasive carcinoma | 328 (84.10) |
aInterval: from core needle biopsy to breast MRI
4.2. Image and Clinical Analyses
As presented in Table 2, subareolar ductal pre-contrast hyperintensity and intra-lesional pre-contrast hyperintensity on T1WI were observed more frequently in cases with non-mass enhancement compared to cases with mass enhancement on DCE-MRI (P < 0.05). Subareolar ductal pre-contrast hyperintensity on T1WI was observed more frequently in cases of ductal carcinoma in situ (DCIS) compared with invasive ductal carcinoma based on surgical results (P < 0.05). Trabecular thickening findings on T2WI correlated with larger lesions based on surgical results (P < 0.05) (Table 2).
| T1, Subareolar Ductal Hemorrhage | P Value | T1, Intra-Lesional Hemorrhage | P Value | T2, High, Hemorrhage or Cystic Change | P Value | T2, Edema | P Value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n = 355) | Positive (n = 35) | Negative (n = 356) | Positive (n = 34) | Negative (n = 385) | Positive (n = 5) | Negative (n = 355) | Positive (n = 35) | |||||
| Age | 51.7 ± 10.9; 50 (24 - 79) | 52.4 ± 8.8; 52 (28 - 67) | 0.4613 | 51.9 ± 10.7; 51 (24 - 79) | 50.4 ± 10.1; 49.5 (34 - 70) | 0.4646 | 51.7 ± 10.6; 51 (24 - 79) | 54.8 ± 15.9; 58 (31 - 71) | 0.4708 | 51.9 ± 10.4; 51 (24 - 79) | 50.0 ± 13.0; 50 (27 - 73) | 0.3923 |
| Tumor Size (cm) | 2.6 ± 1.9; 2.1 (0 - 12) | 3.4 ± 2.4; 2.5 (0 - 9) | 0.1413 | 2.6 ± 1.9; 2.1 (0 - 12) | 3.6 ± 2.6; 2.8 (0.6 - 9.2) | 0.059 | 2.7 ± 1.9; 2.1 (0 - 12) | 3.4 ± 3.2; 1.5 (1 - 7.9) | 0.8699 | 2.6 ± 0.9; 2.1 (0 - 12) | 3.8 ± 2.3; 2.9 (1.2 - 10.5) | 0.0002 |
| Intervala (day) | 13.3 ± 7.8; 12 (0 - 89) | 15.6 ± 9.4; 14 (5 - 50) | 0.1549 | 13.2 ± 7.7; 12 (0 - 89) | 16.7 ± 10.5; 13.5 (5 - 50) | 0.0522 | 13.4 ± 8.0; 12 (0 - 89) | 16.0 ± 6.0; 18 (8 - 23) | 0.2390 | 13.8 ± 8.2; 12 (0 - 89) | 10.4 ± 4.5; 11 (1 - 23) | 0.0091 |
| Operation Method | 0.1216 | 0.3600 | 0.6501 | 0.0071 | ||||||||
| Breast-Conserving Therapy | 248 (69.86) | 20 (57.14) | 247 (69.38) | 21 (61.76) | 265 (68.83) | 3 (60.0) | 251 (70.70) | 17 (48.57) | ||||
| Mastectomy | 107 (30.14) | 15 (42.86) | 109 (30.62) | 13 (38.24) | 120 (31.17) | 2 (40.0) | 104 (29.30) | 18 (51.43) | ||||
| DCE-MRI | 0.0001 | 0.0003 | 0.9999 | 0.9653 | ||||||||
| Mass | 284 (80.0) | 18 (51.43) | 284 (79.78) | 18 (52.94) | 298 (77.40) | 4 (80.0) | 275 (77.46) | 27 (77.14) | ||||
| Non-Mass | 71 (20.0) | 17 (48.57) | 72 (20.22) | 16 (47.06) | 87 (22.60) | 1 (20.0) | 80 (22.54) | 8 (22.86) | ||||
| Cancer Type | 0.0018 | 0.4337 | 0.1807 | 0.0842 | ||||||||
| DCIS | 50 (14.08) | 12 (34.29) | 55 (15.45) | 7 (20.59) | 60 (15.58) | 2 (40.0) | 60 (16.90) | 2 (5.71) | ||||
| Invasive Carcinoma | 305 (85.92) | 23 (65.71) | 301 (84.55) | 27 (79.41) | 325 (84.42) | 3 (60.0) | 295 (83.10) | 33 (94.29) | ||||
Abbreviations: DCE, dynamic contrast enhanced; DCIS, ductal carcinoma in situ.
aInterval: from core needle biopsy to breast MRI.
4.3. Histopathological Analysis
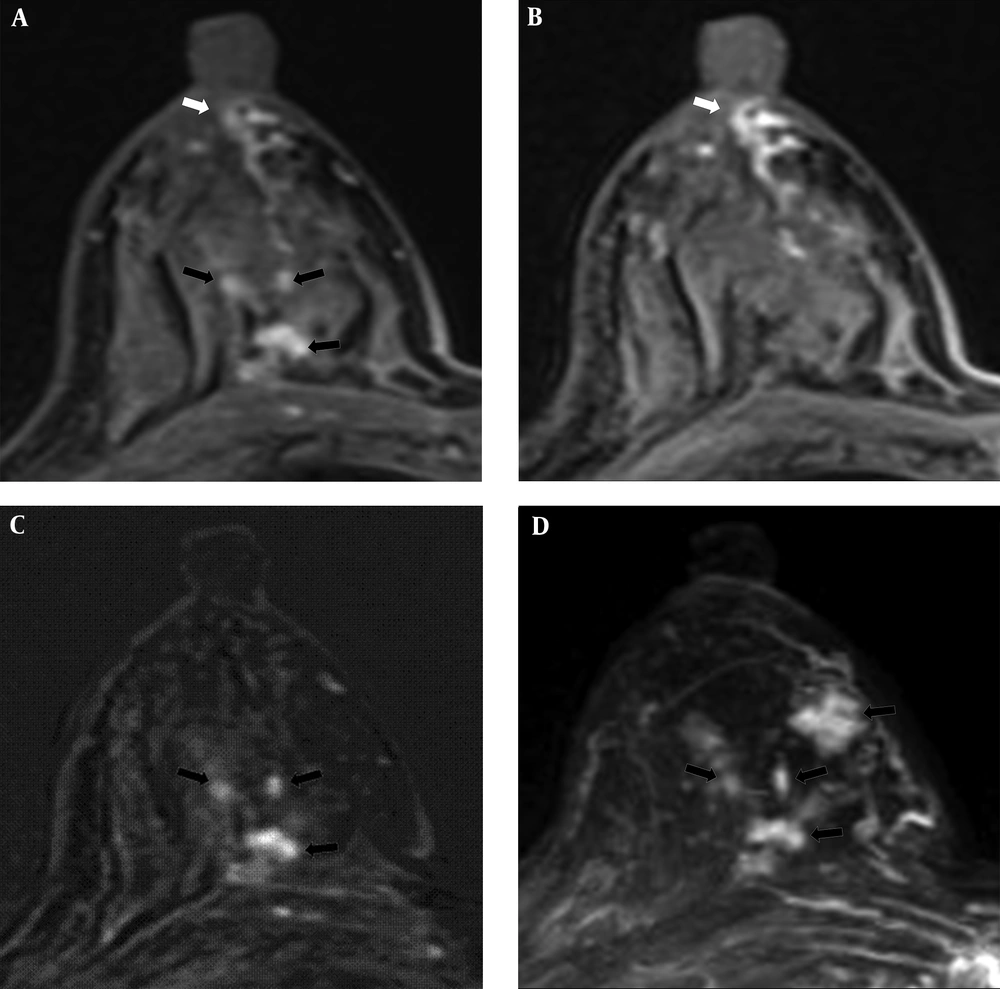
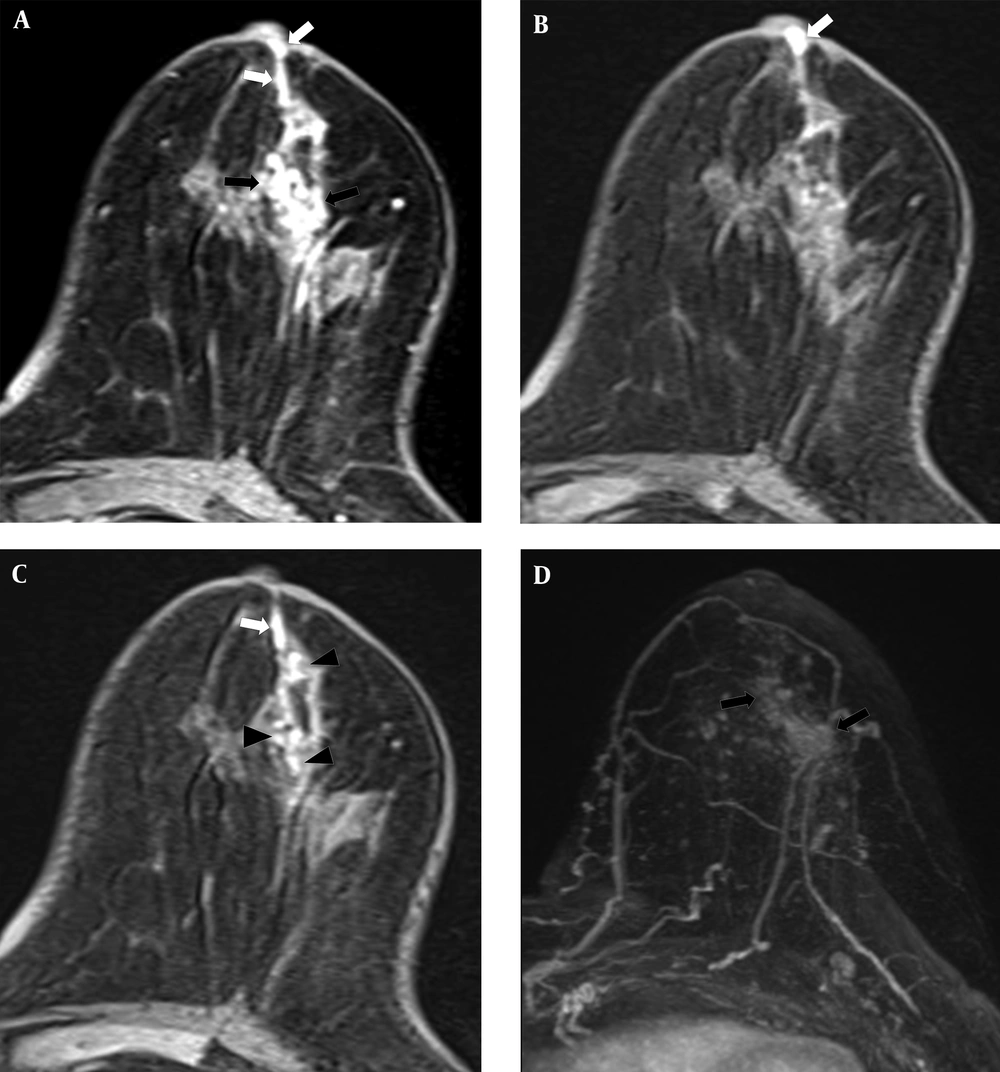
Two of the 35 cases with subareolar ductal high signals on the pre-contrast T1WI were confirmed to have nipple-areolar complex involvement upon surgery. As presented in Table 3, these two cases exhibited multiple enhancing masses on DCE-MRI or subtle enhancement around the subareolar duct on DCE-MRI. Four invasive carcinomas with 4 cm DCIS lesions were noted in one case, and two 1.5 cm DCIS lesions were identified in the other case upon surgery (Figure 1). No nipple-areolar complex involvement was detected in the remaining 33 cases (Figure 2).
| No. | Age | Pre-Contrast MRI | Contrast-Enhanced MRI | Size in MRI | Size in Operation | Operation | Biopsy Pathology (× number) (size) | Subareolar Involvement | Inta |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | SD | Masses; irregular, spiculated, heterogeneous | 2.5 | 4 | SM | IDC (×4), DCIS (4 cm) | + | 23 |
| 2 | 54 | SD | Masses; oval, smooth, heterogeneous | 1.8, 0.8 | 3 | SM | No IDC, DCIS (1.5 cm, 1.5 cm), | + | 26 |
Abbreviations: DCIS, ductal carcinoma in situ; IDC, invasive ductal carcinoma; MD, (moderate differentiated); PD, (poorly differentiated); SD, subareolar ductal pre-contrast high signal on T1WI; SM, simple mastectomy; WD (well differentiated).
aInt: Interval period from core needle biopsy to breast MRI.
A 54-year-old woman had 1.8 cm and 0.8 cm lesions in the right breast that were confirmed as ductal carcinoma in situ by core needle biopsy. Subareolar involvement was confirmed on the pathological report after mastectomy. A, A post-contrast enhanced image revealed multiple enhancing lesions (black arrows) with a subareolar ductal high signal area (white arrow) that was described as BI-RADS category 6. B, A pre-contrast enhanced T1WI exhibiting subareolar ductal pre-contrast high signal area (white arrow). No findings were detected on T2WI. C, Subtraction and D, Maximal intensity projection images reveal multiple enhancing lesions (black arrows).
A 67-year-old woman had a 7 cm lesion in the left breast that was confirmed to be invasive ductal cancer and ductal carcinoma in situ by core needle biopsy. Subareolar involvement was not confirmed on the pathological report after mastectomy. A, A post-contrast enhanced image revealing segmental non-mass enhancement (black arrows) with subareolar ductal high signal areas (white arrows) was described as BI-RADS category 6. B and C, A pre-contrast enhanced T1WI exhibiting subareolar ductal (white arrows) and intra-lesional pre-contrast high signal areas (black arrowheads). No findings were detected on T2WI. D, Maximal intensity projection reveals segmental non-mass enhancement (black arrows) without subareolar ductal enhancement.
4.4 Relationship Between Findings and Interval Period from Core Needle Biopsy
In the pre-contrast T1WI and T2WI, all non-enhancing findings associated with post-core needle-biopsy changes disappeared over time. On the T2WI, a significant interval change of edema was noted from the time from core needle biopsy to breast MRI (Tables 2 and 4). Two cases of subareolar involvement were included at the fourth week based on core needle-biopsy result and breast MRI findings (Table 4).
| 1 Week (0 - 7 days) | 2 Weeks (8 - 14 days) | 3 Weeks (15 - 21 days) | 4 Weeks (22 - 28 days) | 5 Weeks (29 days) | P Value | |
|---|---|---|---|---|---|---|
| Distribution of Patients Based on Interval Period (%) | 50 (12.92) | 209 (54.01) | 90 (23.26) | 23 (5.94) | 15 (3.88) | |
| T1, Subareolar Ductal Hemorrhage | 0.4706 | |||||
| Negative | 48 (96.0) | 191 (91.39) | 80 (88.89) | 20 (86.96) | 13 (86.67) | |
| Positive | 2 (4.0) | 18 (8.61) | 10 (11.11) | 3 (13.04)b | 2 (13.33) | |
| T1, Intra-Lesional Hemorrhage | 0.0483 | |||||
| Negative | 49 (98.0) | 191 (91.39) | 80 (88.89) | 22 (95.65) | 11 (73.33) | |
| Positive | 1 (2.0) | 18 (8.61) | 10 (11.11) | 1 (4.35) | 4 (26.67) | |
| T2, High, Hemorrhage or Cystic Change | 0.3840 | |||||
| Negative | 50 (100.0) | 207 (99.04) | 88 (97.78) | 22 (95.65) | 15 (100.0) | |
| Positive | 0 (0.0) | 2 (0.96) | 2 (2.22) | 1 (4.35) | 0 (0.0) | |
| T2, Edema | 0.0342 | |||||
| Negative | 40 (80.0) | 189 (90.43) | 86 (95.56) | 22 (95.65) | 15 (100.0) | |
| Positive | 10 (20.0) | 20 (9.57) | 4 (4.44) | 1 (4.35) | 0 (0.0) |
aValues are presented as No. (%).
bTwo subareolar involvement cases were included at 4 weeks.
5. Discussion
The clinical importance of breast MRI has increased since it was first clinically applied. One of the main advantages of breast MRI is its high sensitivity in detecting breast cancer (1, 2). However, the specificity of breast MRI is low due to the overlapping features of benign and malignant breast lesions (17-19). Diagnostic criteria based on contrast-enhanced breast MR images to differentiate benign and malignant lesions include both morphologic and kinetic features (20). Breast MRI, including all series of DCE-MR images, could also provide us with more diagnostic information about breast lesions (21). In terms of non-mass enhancement lesions, malignancies might be strongly differentiated from benign lesions using preoperative 3-T DCE-MRI, including additional non-mass enhanced linear or segmented lesions in patients with recently diagnosed breast cancer (22).
The choice between BCT and mastectomy depends on numerous factors, including tumor size, location, grade, tumor size to breast volume ratio, multifocality or multicentricity, and patient preference (23, 24). Breast MRI positively affects patient management and is recommended for mapping tumor extent in patients with newly diagnosed cancer (5). MRI detects more cases of multifocal, multicentric, and contralateral disease than other modalities and leads to changes in the treatment plan in approximately 25% of cases (5).
The Breast Imaging Reporting and Data System (BI-RADS) lexicon includes terms used to describe morphological characteristics and kinetic features of breast lesions and define the final assessment categories to describe the level of suspicion regarding MR findings. Several studies have evaluated the positive predictive value (PPV) of the BI-RADS assessment categories in contrast-enhanced breast MRI imaging to identify the BI-RADS MRI imaging lesion features that are most predictive of cancer (20, 25-27). However, only a few studies on other descriptors of post-biopsy changes on pre-contrast MRI are available. Even after publication of the 5th American College of Radiology BI-RADS MRI lexicon, no reports have been published regarding characteristic findings on pre-contrast MRI, including post-biopsy changes.
The characteristics of findings associated with core needle biopsy in the BI-RADS lexicon regarding pre-contrast T1WI and T2WI are as follows: a) subareolar ductal pre-contrast high signal on T1WI indicates subareolar ductal hemorrhage; b) intra-lesional pre-contrast high signal on T1WI indicates intra-lesional hemorrhage; c) pre-contrast high signal on T2WI indicates hemorrhagic or cystic changes; and d) trabecular thickening on T2WI indicates subcutaneous/peritumoral edema.
In a study about the PPV evaluation of BI-RADS MR images, one case was defined as BI-RADS categories 0, 3, 4, and 5 due to its pre-contrast ductal high-signal intensity (20). No other report has evaluated similar pre-contrast MRI findings.
Mastectomy is the treatment of choice for patients with a presumed risk of nipple-areolar complex involvement. Nipple-areolar complex involvement has been reported in 5.6% - 24.6% of breast cancer patients (28). The preoperative 3-T MRI is known as a useful method to predict nipple-areolar complex involvement (28).
In our study, the subareolar ductal hyperintensity on T1WI was the only meaningful finding among the characteristic findings associated with core needle biopsy in the BI-RADS lexicon of pre-contrast T1WI and T2WI. In cases with a subareolar ductal high signal on pre-contrast T1, the mean distance from biopsy site to areolar was 1.9 ± 1.2 cm under MRI in this study. Additionally, subareolar ductal hemorrhage was described as subareolar ductal high signal intensity on pre-contrast T1WI and mimicked subareolar involvement on DCE-MRI (Figures 1A and 2A).
The subareolar ductal pre-contrast hyperintensity was observed more frequently in cases with non-mass enhancement than in cases with mass enhancement on DCE-MRI (P < 0.05). Subareolar ductal pre-contrast hyperintensity on T1WI was observed more frequently with DCIS compared with invasive ductal carcinoma based on surgical results (P < 0.05).
Ultrasonography-guided core needle biopsy is the standard tool for confirming breast cancer at our institution. Mammography-guided vacuum-assisted biopsy is performed for lesions exclusively detected by mammography, and MRI-guided vacuum-assisted biopsy is performed for lesions exclusively detected by MRI. Patients who underwent vacuum-assisted or excisional biopsy were excluded, and only core needle biopsy cases were included in this study. We evaluated the interval from core needle biopsy to breast MRI. All non-enhancing findings associated with core needle biopsy on pre-contrast T1WI and T2WI disappeared over time. Two subareolar involvement cases were included at the fourth week, which were based on core needle biopsies from breast MRI cases. Thus, we should be concerned that the subareolar ductal pre-contrast high signal on T1WI remains consistent for 4 weeks. However, further evaluation is required to analyze the biopsy changes and interval.
Our study had several limitations. First, it was a retrospective study with a small sample. Furthermore, the interval from biopsy to MRI was not contiguous. A prospective study with a larger sample size is required to validate our results. In addition, interobserver and intraobserver reliability were not evaluated in this study. Experience with breast MRI could be crucial for determining the clinically significant findings on pre-contrast images. Finally, post-biopsy change findings were not confirmed, and we were unable to differentiate post-biopsy changes from cancer findings, such as blood discharge from the nipple, cancer hemorrhage, or edema with mass effect. However, this is the first investigation that evaluated pre-contrast MRI features of the breast using the updated BI-RADS lexicon.
In conclusion, pre-contrast T1WI and T2WI images rarely exert a clinical impact. However, a subareolar ductal high signal finding on DCE-MRI mimicking subareolar cancer involvement correlated with pre-contrast T1WI. Usually subareolar ductal high signal intensity on T1WI without enhancement is not meaningful for surgical planning. However, a persistent subareolar ductal pre-contrast high signal on T1WI and subtle enhancement around the subareolar duct should be carefully evaluated, particularly for non-mass-enhanced or multifocal-enhanced masses due to DCIS. Therefore, a subareolar ductal high signal intensity on a pre-contrast T1WI must be carefully assessed in combination with preoperative dynamic contrast-enhanced images for proper surgical management.