1. Background
Multiple sclerosis (MS) is a central nervous system (CNS) myelogenous disease and is still the most common immune-mediated disorder affecting the CNS. The onset of MS is most commonly seen in people aged 20 to 40 years (1). Pathophysiological processes in MS patients include chronic inflammation, oxidative stress, and axonal demyelination (2, 3). The myelin destruction of axons is revealed by blocking the impulse conduction and the process of permanent inflammation (4).
Although the pathogenesis of MS is not yet fully understood, CNS axonal damage is likely to be directly related to the exacerbation of inflammatory processes and oxidative stress (5). According to evidence, a lot of inflammatory and oxidative stress mediators, including cytokines and chemokines, are secreted in MS. On the other hand, it has been indicated that oxidative stress is involved in inflammatory and autoimmune processes. Therefore, antioxidant therapy could help reduce the progression of MS (6).
Cytokines represent as critical mediators of the autoimmune process. Studies show a correlation between MS symptoms and increased production of inflammatory cytokines (7). Accordingly, cytokine expression is elevated in serum, cerebrospinal fluid, and cultured mononuclear cells of MS patients. Some of these augmented cytokines include tumor necrosis factor (TNF), interferon γ (IFN-γ), interleukin 12 (IL-12), and interleukin 1 (IL-1) (8, 9).
Produced by T-helper cells, IFN-γ has several effects, such as the induced expression of major histocompatibility complex (MHC).
Many of these features happen in MS brain lesions and could thus show a relationship between this cytokine and the disease (10). The other types of cytokines that participate in this regulation are anti-inflammatory cytokines such as transforming growth factor (TGFβ), IL-10, and IL-4. As known, IL-10 is the most important anti-inflammatory cytokine in the human immune system. After connecting to its receptor, it inhibits monocyte/macrophage pro-inflammatory cytokine production, including IFN-γ and TNF-α (11-13).
Olive is a fruit rich in flavonoids. One of the most important flavonoids extracted from olive is hydroxytyrosol l (HT) (3,4-dihydroxyphenyl)ethanol, with antioxidant and anti-inflammatory activities. Besides, HT has been detected in the olive pulp, vegetation water, and pomace; however, it has not been found in virgin olive oil, as shown in previous work (14-16).
2. Objectives
This study aimed to evaluate the probable inflammatory or anti-inflammatory effects of olive vegetation water (OVW) on peripheral blood mononuclear cells (PBMCs) collected from MS patients. We chose IFN-γ as a pro-inflammatory and IL-10 as an anti-inflammatory cytokine, which works in the opposite direction.
3. Methods
3.1. Subject Selection
In this study, 20 MS patients (aged 32 ± 14 years) were selected among those referring to Ganjavian Hospital in Dezful city (Khuzestan province, Iran) between April 2016 and June 2018 based on their medical history and status, confirmed by specialist physicians according to the clinical findings and McDonald criteria (17). Peripheral blood samples were collected before receiving any therapy. Patients with other disorders such as liver diseases, alimentary tract, infections, and other complications were excluded from the study. This study was evaluated and approved by the Ethics Committee of Dezful University of Medical Sciences.
3.2. Sample Preparation and Cell Culture
Blood samples were collected after 8 to 10 hours of fasting in heparinized tubes containing 200 IU/mL of heparin (Sigma Chemical Co., Munich, Germany). Then, PBMCs were isolated by using Ficoll-Hypaque gradient centrifugation (Sigma Chemical Co., Munich, Germany). Washing of the isolated cells was done by PBS, and they were resuspended in RPMI 1640 medium (Gibco) containing 10% heat-inactivated FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin (Gibco), cultured at 37°C in a humidified 5% CO2 incubator as indicated before (18-21). Next, a density of 2 × 105 cells/well of suspensions was prepared in a 96-well microplate. Finally, PBMCs from each patient were treated with OVW for 48 h and 72 h.
3.3. Solutions and Reagents
First, Taarom olive fruit, collected in the 2015 - 2016 crop year, was pitted, and then the seedless pulp was mechanically pressed to give a liquid phase mixture including olive oil, vegetation water, and solids. The solids were separated from the liquid phase mixture by filtration and olive oil, and water fractions were separated by centrifugation at 8,000 g for 40 min. Then, PBMCs were incubated with 0, 10, 20, or 50 µL of the extracted aqueous fraction for 48 h and 72 h.
3.4. Cytotoxicity Assay
The cytotoxicity of OVW on PBMCs in MS patients was examined using a standard MTT assay with 4, 5-dimethylthiazoyl-2, 5-diphenyltetrazolium bromide (Sigma). In this stage, 5 × 104 cells were cultured in the presence of 0, 10, 20, or 50 µL of OVW for 72 h. After the incubation time passed, 10 µL of the MTT reagent (0.5 mg.mL-1) was added to each well and incubated for 4 h. Then, 100 µL of acidified isopropanol was added to the wells. After the generation of purple formazan crystals, the absorbance of the samples was measured using an ELISA reader (Awareness, USA) at 570 nm and 630 nm as the reference wavelength.
3.5. Cytokine Measurement
After incubating, the supernatants were aspirated. Then, using the sandwich ELISA (Quantikine, R&D Systems, Minneapolis, MN) technique, the concentrations of INF-γ and IL-10 were measured. Standard curves were generated at the same time with each set of samples. All the tests were performed in duplicate.
3.6. Statistical Analysis
Statistical analyses were performed using SPSS (version 19). One-way ANOVA and student t-test were used to check for statistical differences in INF-γ and IL-10 secretions in the presence and absence of OVW. The results are presented as mean ± SD, and the level of significance was considered as P value < 0.05.
4. Results
The viability percent of PBMCs, measured by the MTT assay, after co-culture with OVW (10, 20, or 50 µL) did not change in comparison with the control (all concentrations showed more than 97.5% viability compared to the control at 72 h incubation time). Thus, OVW had no detrimental effect on PBMCs viability at the concentrations used for treatment in this study.
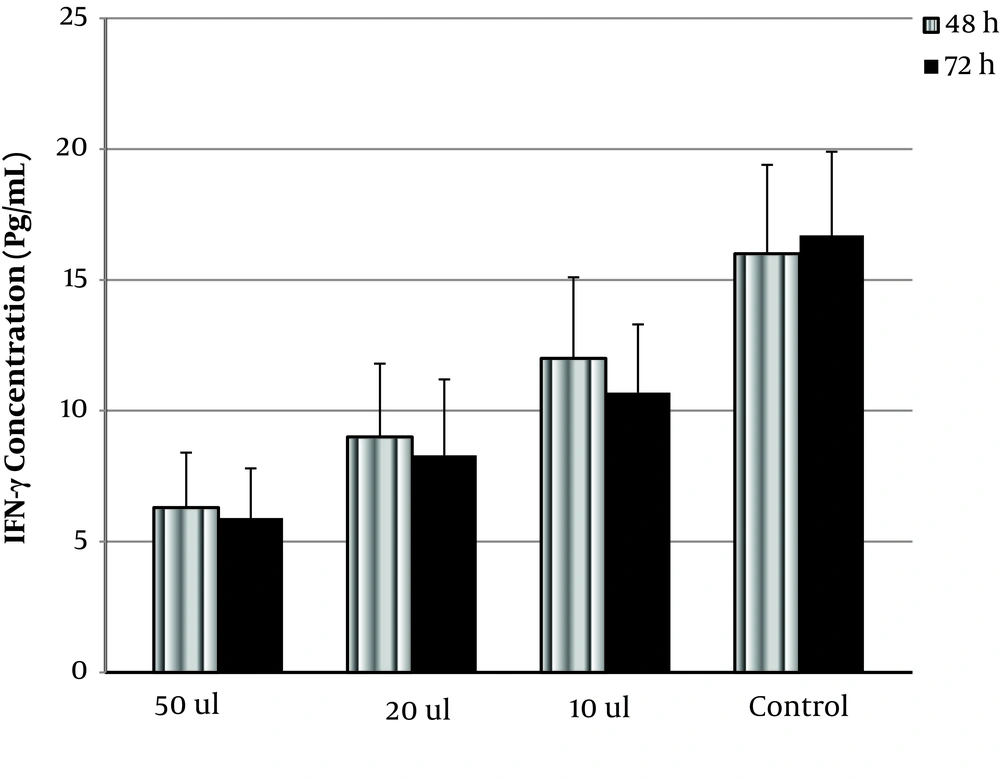
Also, INF-γ and IL-10 concentrations were tested by ELISA. As shown in Figure 1, OVW significantly decreased IFN-γ dose-dependently (P < 0.05). The amount of secreted IFN-γ in the presence of 10, 20, and 50 µL of OVW was 10.7 ± 2.6, 8.3 ± 2.3, and 5.9 ± 1.9 pg/mL (compared to control with 16.7 ± 3.2 pg/mL) in 72 h, respectively. The difference between the effect of OVW on INF-γ secretion at 48 h and 72 h was not significant.
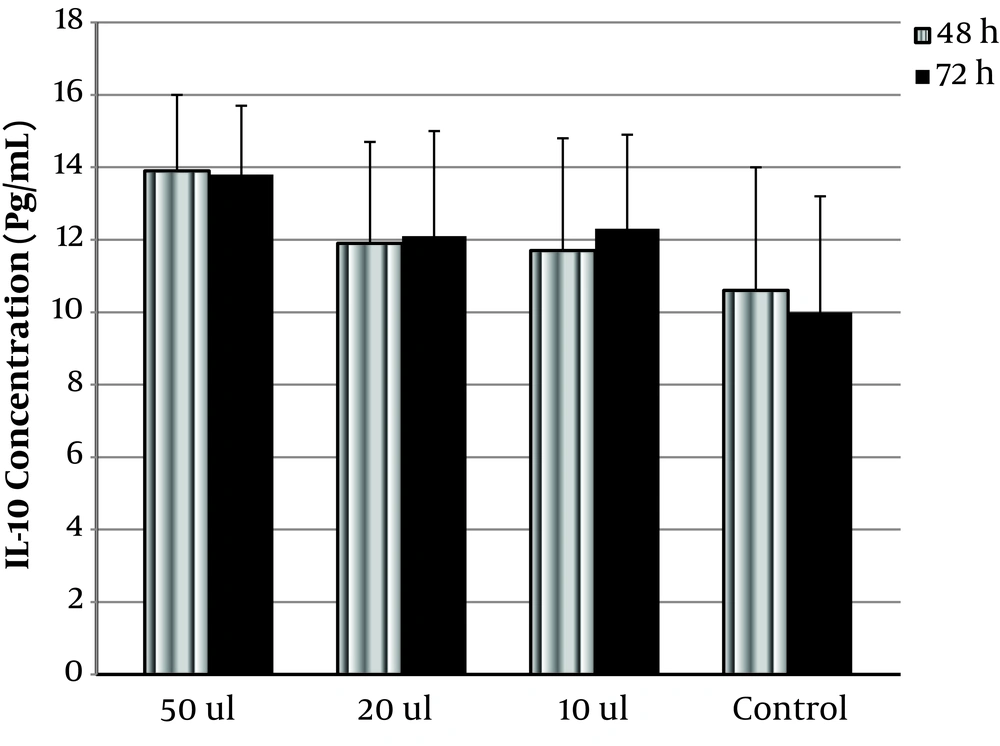
OVW at 10, 20, and 50 µL increased IL-10 significantly from 10 ± 3.2 to 12.3 ± 2.6, 12.1 ± 2.9, and 13.8 ± 1.9 pg/mL, respectively (P < 0.05) (Figure 2). The difference between the effect of OVW on IFN-γ and IL-10 secretion at 48 h and 72 h was not significant.
5. Discussion
MS is one of the most common autoimmune diseases among young people. The pathological process is still unknown, which makes it more difficult to find a cure. There is much research investigating new ways and compounds to exert an anti-inflammatory effect on the disease. The autoimmunity concept has lead studies to work on the immune system and suppressing the pro-inflammatory cytokines. The immune pathway cytokines that work in are complicated and partly unknown, so further studies are necessary to find out how they can affect the disease process.
The same happens for olive products and OVW that was used in this study. A decrease in IFN-γ, a pro-inflammatory cytokine, showed the anti-inflammatory effect of OVW. The effect was seen dose-dependently, which showed a direct correlation between the concentration of OVW and the reduction of IFN-γ. On the other hand, the treatment caused a significant increase in IL-10. As known, IL-10 is a cytokine that suppresses the effect of pro-inflammatory cytokines, making it an anti-inflammatory cytokine. Increasing IL-10 can be a sign of suppressing the inflammatory condition in MS patients. It is in agreement with data published by Bitler et al., which showed OVW had anti-inflammatory effects in addition to antioxidant activity (22).
There are several polyphenols with potential health benefits in olive fruit that are retained in the olive vegetation wastes during virgin olive oil processing. Some of them, due to their hydrophilic properties, are concentrated in OVW. Among them, 3,4-dihydroxyphenethyl alcohol (hydroxytyrosol) has been well recognized for its health beneficial activities, including antioxidant and anti-inflammatory activities. It should be noted that the concentration of hydroxytyrosol is more than 10 times higher in OVW than in the corresponding virgin olive oil due to its high hydrophilic properties. Therefore, hydroxytyrosol, as one of the main known constituents of OVW, could be the reason for the anti-inflammatory effect of OVW (23-26).
Although in this study, we demonstrated the effects of the aqueous olive extract on PBMCs obtained from MS patients, it would be good to continue the study by employing healthy controls and/or patients with other immune-mediated diseases. Furthermore, to confirm the anti-inflammatory effects of OVW, we propose to further investigate the effect of different polyphenols extracted from OVW by testing the above-mentioned cytokines and some other ones. This study could be a starting point for future studies on the effects of the aqueous extract of olive on the cytokine profile of MS patients.