1. Background
Breast cancer (BC) is the most common malignant tumor in women around the world, with 2,088,849 new cases diagnosed and accounting for 25.4% of all cancers, excluding non-melanoma skin cancer in 2018 (1). Similar to other cancers, genetic factors play a significant role in the development and progression of BC (2). Previous studies showed that around 70% of diagnosed BC cases express estrogen receptor alpha (ERα); on the other hand, ER signaling can lead to tumor growth and progression in ER-positive BC (3). The ESR1 (estrogen receptor 1) gene is located at 6q25.1-q25.2 and encodes a transcription factor containing activation function 1 (AF1) domain, DNA-binding domain (DBD), ligand-binding domain (LBD), and ligand-dependent activation function 2 (AF2) domain (3). Khan et al. (4) reported that expression of the ER is associated with a genetic predisposition to BC. Long-term exposure of breast tissue with high levels of estrogen by stimulating the ER leads to a defective estrogen-mediated gene expression and may result in cancer. A study by Khakpour et al. (5) showed the carcinogenesis of the ESR1 in breast tissue by epigenetic mechanisms. Indeed, methylation of the ESR1 may affect the activity of normal tissue in the breast.
Single nucleotide polymorphisms (SNPs) in the ESR1 gene are related to the cancerous tumor, cell proliferation, and metastasis (6). Madeira et al. (7) evaluated the rs2234693 and rs9340799 SNPs in 64 formalin-fixed, paraffin-embedded tissue samples of primary BC compared to 72 blood samples from healthy women as a control group. Genotyping for these SNPs was performed by polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method and confirmed by Sanger sequencing. A significant difference in the rs2234693 allele frequency was observed with 5.14-fold increased BC risk. Previous studies have shown an increased risk of BC in European and African American women related to the rs1801132 (8); however, there is some contradictory evidence in other studies, which may be due to differences in sample size and genetic diversity. The results of a study by Li and Xu (9) showed that the rs2234693 C>T SNP is a risk factor for BC in pre-menopausal women.
Several studies have shown the interdependence between SNPs in the ESR1 gene and genetic predisposition to BC. Nevertheless, due to genetic diversity, the data of different studies in this regard is contradictory and controversial. Therefore, in the current study, we aimed to investigate the potential relationship between the rs1801132 and rs2234693 SNPs of the ESR1 gene with susceptibility to BC in the Iranian population.
2. Objectives
This research aimed to examine the relationship between the rs1801132 and rs2234693 SNPs of the ESR1 gene with susceptibility to BC in the Iranian population.
3. Methods
3.1. Patients and Sampling
A total of 63 BC patients were recruited from the Imam Hasan Mojtaba Center, which is a charity-based foundation for cancer care in Dezful, Iran, from March 2018 to November 2019. Moreover, 65 age- and sex-matched healthy women were selected as a control group. The control group had no type of cancer and were unrelated to the patients. After completing an informed consent form by patients or their families, the age and stage of cancer were collected. The Review Board of Dezful University of Medical Sciences approved this study (ethical code: IR.DUMS.REC.1397.022).
3.2. Blood Sampling and DNA Extraction
In the present study, 2 mL of peripheral blood was obtained from both groups, then added to the EDTA tubes and stored at -20°C until DNA extraction. DNA was extracted using Prime Prep Genomic DNA Isolation Kit (GeNet Bio, Korea). The quality and purity of DNA were evaluated by agarose gel electrophoresis and NanoDrop® ND-1000 UV-Vis Spectrophotometer (Thermo Fisher Scientific, Waltham, MA USA), respectively.
3.3. Genotyping and DNA Sequencing
The genotyping of the rs1801132 C>G and the rs2234693 T>C SNPs of the ESR1 gene was performed using the High Resolution Melting (HRM) technique by LightCycler® 96 Instrument (Roche Life Science). Each PCR reaction consisted of 4 µL of 5x HOT FIREPol® EvaGreen® HRM Mix (Solis biodyne), 1 µL of each primer, 2 µL (100 ng) of genomic DNA, and 12 µL of DNase/RNase free water. (Final volume = 20 µL). The thermal cycling program was set to a schedule as follows: pre-incubation at 95°C for 5 min; 40 cycles at 95°C, 61°C, and 72°C each one for 30 s; and finally, HRM at 95°C for 60 s, at 40°C for 60 s, at 65°C for 1 s, and at 97°C for 1 s continuously.
The PCR and DNA sequencing were done on 10 samples of each genotype (total of 30 PCR products) to confirm the accuracy of the HRM results. DNA direct sequencing was completed with ABI 3130XL capillary sequencing platform (Applied Biosystems/Life Technologies, Carlsbad, CA, USA). The primer sequences for the rs1801132 and rs2234693 SNPs were designed using the Gene Runner V.4.0 and primer3 V.4.1.0 software (Table 1).
| SNP | Forward Primers | Revers Primers |
|---|---|---|
| rs1801132 | 5’CTCATGATCAAACGCTCTAAGA3’ | 5’ATCATGTGAACCAGCTCCC3’ |
| rs2234693 | 5’TGCTCAGTCTCTACATGTTCCT3’ | 5’ACCAATGCTCATCCCAACT3’ |
3.4. Statistical Analysis
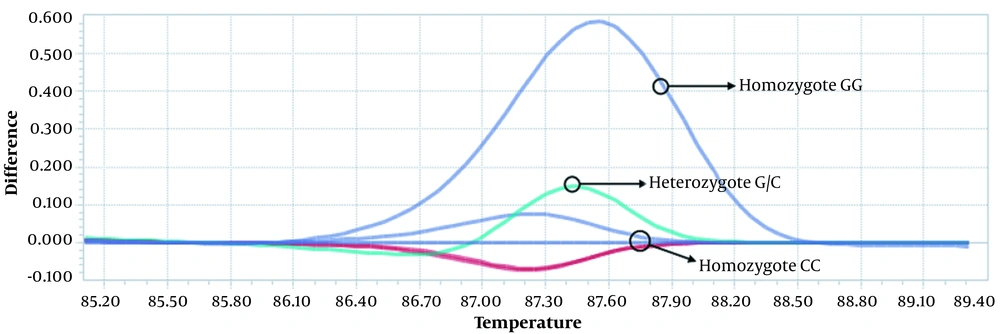
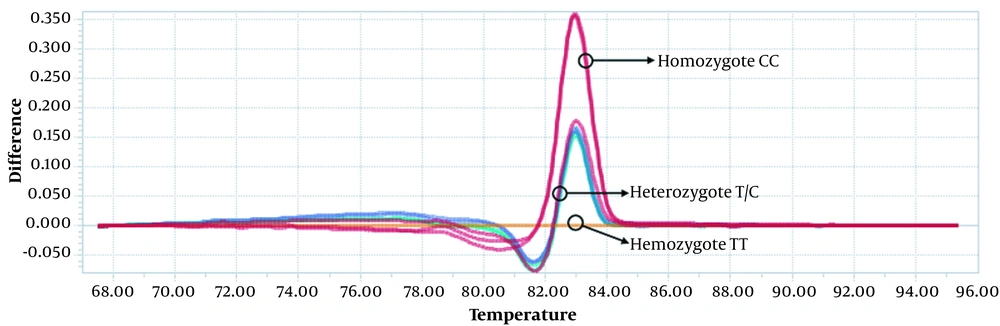
Statistical analysis was performed using the SPSS software (version 22.0, SPSS Inc., Chicago, IL, USA). The Hardy-Weinberg equilibrium (HWE) was assessed in the patient and control groups using the χ2 test (P < 0.05). The odds ratios (OR) and 95% confidence intervals (CI) were considered to assess the relationships between ESR1 polymorphisms and BC risk using χ2 test. The multiple inheritance models (dominant, recessive, and codominant) were done using χ2 test. The two-sided P-value of less than 0.05 was considered as statistically significant. The genotyping was performed by LightCycler® 96 Software v.1.1.0.1320 and the difference plot method. This graph is obtained by subtracting the normalized fluorescence data from the temperature of each sample relative to the baseline sample (a predefined genotype). This graph actually highlights the differences between similar melt curves. The DNA sequencing results were obtained as the electropherograms and evaluated using the chromas V. 2.6.6.
4. Results
4.1. Epidemiological and Clinicopathologic Results
A total of 63 BC patients and 65 healthy individuals participated in this study. The mean age was 46.3 ± 4.8 years in patients and 47.1 ± 3.6 years in controls. There was no significant difference in age distribution between case and control groups (P = 0.407). All patients were female with at least one pregnancy; thus, there was no gender difference between the groups. Out of 63 BC patients, 21 (33.3%) patients were in the early TNM (Tumor, Node, Metastasis) stage (I, II), and 42 (66.6%) patients were in the late stages (III, IV).
4.2. Association Analysis of the rs1801132 and rs2234693 Polymorphisms in BC Patients
The control group was in the Hardy-Weinberg Equilibrium (HWE). The differences in genotype frequency and allele frequency between patients and healthy control were compared by χ2 test. The genotype frequency (P = 0.772 for the rs1801132 and P = 0.018 for the rs2234693) and allele frequency (P = 0.893, OR = 1.034, CI 95% = 0.633 - 1.689 for the rs1801132 and P = 0.004, OR = 2.085, CI 95% = 1.253 - 3.468 for the rs2234693) related to the rs2234693 were significantly different between patients and the control group (Table 2). Due to the significant association of the rs2234693 SNP with BC, subsequent analyses were performed on this polymorphism. While the genotype frequency of the TT, TC, and CC in the patient group was 19%, 25.4%, and 55.6%, respectively, it was 29.2%, 40%, and 30.8% in the control group, respectively. The frequency of the allele C was higher in the BC group than in controls (68.2% vs. 50.8%). The effect of each genotype on BC risk was calculated in dominant, recessive, and codominant genetic models. Statistical analysis showed an increased risk of BC in the CC compared to TT (P = 0.026, OR = 2.771, CI 95% = 1.18 - 6.869) and in the recessive model (P = 0.005, OR = 2.813, CI 95% = 1.363 - 5.802) (Table 3).
| SNP | Groups | Genotype Frequency (%) | P-Value | Allele Frequency, % | P-Value | OR | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GG | GC | CC | G | C | ||||||
| rs1801132 | Patients (n = 63) | 17 (27.0) | 32 (50.8) | 14 (22.2) | 0.772 | 52.40 | 47.6 | 0.893 | 1.034 | 0.633 - 1.689 |
| Control (n = 65) | 19 (29.2) | 29 (44.6) | 17 (26.2) | 51.50 | 48.5 | |||||
| TT | TC | CC | T | C | ||||||
| rs2234693 | Patients (n = 63) | 12 (19.0) | 16 (25.4) | 35 (55.6) | 0.018 | 31.8 | 68.2 | 0.004 | 2.085 | 1.253 - 3.468 |
| Control (n = 65) | 19 (29.2) | 26 (40.0) | 20 (30.8) | 49.2 | 50.80 | |||||
| SNP ID | Model | P-Value | OR | 95% Confidence Interval |
|---|---|---|---|---|
| rs2234693 | CC vs. TT | 0.026* | 2.771 | 1.18 - 6.869 |
| TC vs TT | 0.957 | 0.974 | 0.375 - 2.530 | |
| Dominant | 0.179 | 1.755 | 0.769 - 4.007 | |
| Recessive | 0.005* | 2.813 | 1.363 - 5.802 | |
| Codominant | 0.079 | 0.511 | 0.240 - 1.085 |
a, *Bold values indicate statistical significance (P < 0.05).
4.3. HRM Results and DNA Sequencing Analysis
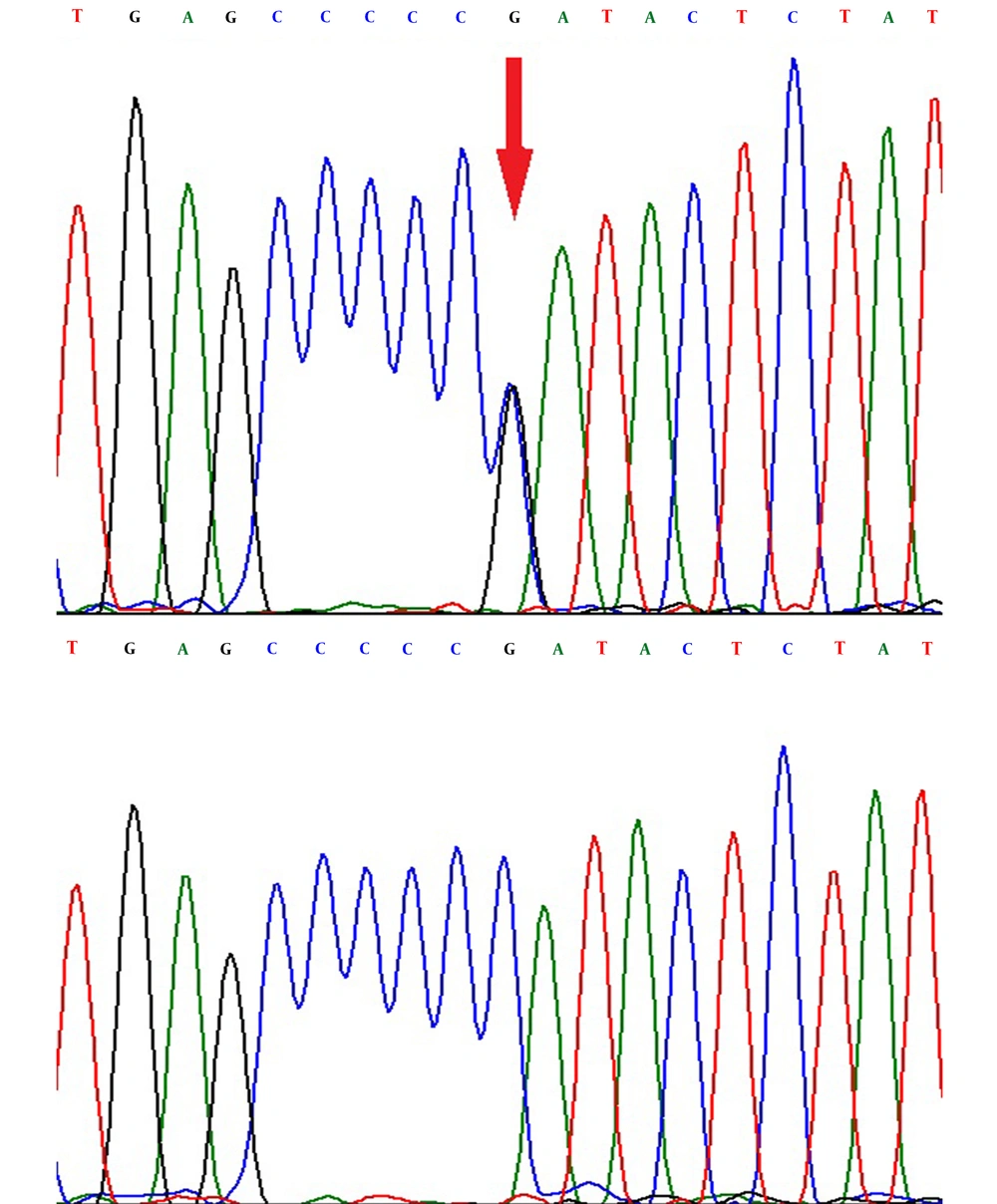
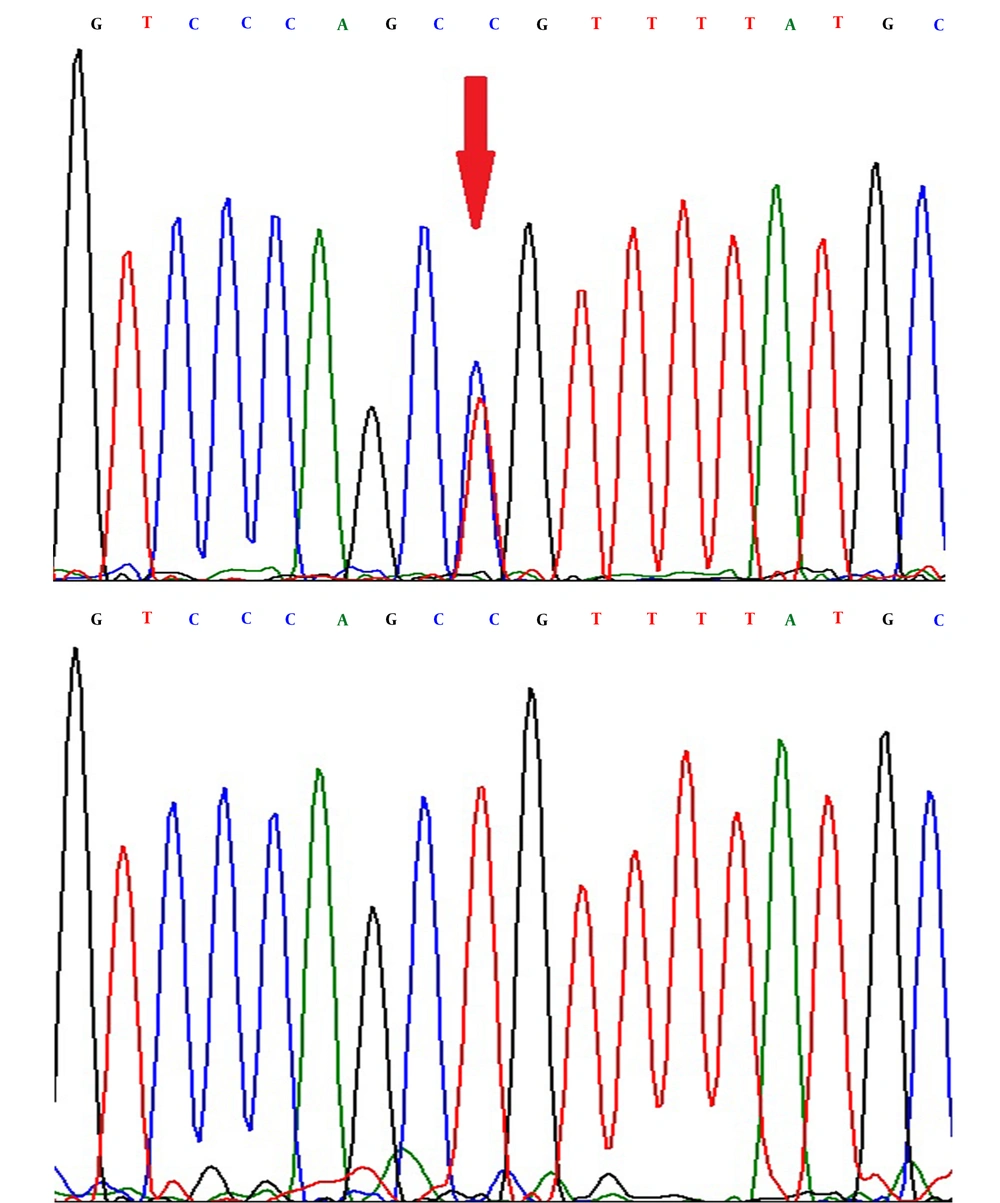
The difference of the curves was obviously detected in the heterozygous and homozygous status in the rs1801132 G>C (Figure 1) and the rs2234693 T>C (Figure 2) SNPs. Also, the DNA sequencing showed the heterozygous and homozygous status in the rs1801132 G>C (Figure 3) and rs2234693 T>C (Figure 4) SNPs.
5. Discussion
The influence of the ESR1 gene disturbance in various cancers such as breast, liver, and prostate has been demonstrated in some studies. The ESR1 protein acts as a transcription factor and is responsible for transcribing important genes, such as regulating proliferation genes. Considerable to the significant role of genetic susceptibility in cancer, scientists have focused on assessing the association of the polymorphisms in hormone receptors, especially estrogen, with BC risk (10). As part of precision medicine, some SNPs can be used for a variety of clinical applications, such as susceptibility to various diseases. Genetic predisposition factors play an important role in BC (11). A study by Zhang et al. (12) reported that genetic variations in the rs2234693 and rs1801132 can lead to estrogen dysfunction and increased risk for BC.
Our results revealed that the CC genotype of the rs2234693 SNP in patients was significantly related to BC. However, we did not find an association between the rs1801132 SNP and BC. These results are consistent with a study conducted by Hu et al. (13), reporting that the CC genotype of the rs2234693 SNP was associated with an increased risk for BC, although there was no correlation between the rs1801132 SNP and BC risk. Therefore, the CC genotype of the rs2234693 SNP in BC patients may help in the diagnosis of patients that are predisposed to BC.
The rs2234693 SNP is an intronic variant, and its effect on the receptor’s function is probably due to incorrect splicing and abnormal expression. Herrington et al. (14) reported that the allele C of the rs2234693 SNP produces a functional binding site for the B-Myb transcription factor and significantly increases the transcription of the downstream constituent compared to the T allele, which may explain its high association with the risk of BC. Cai et al. (15) investigated the genetic SNPs in the ERα gene and BC risk in 1,069 BC patients and 1,166 healthy controls and reported that the rs2234693 SNP increased BC risk among individuals carrying CT and TT genotypes (15). Moreover, Li et al. (16) examined the rs2234693 SNP in 10,300 BC patients and 16,620 healthy controls and showed a borderline significant reduced risk for BC in the CC and CC/CT carriers. Genotyping of the rs1801132 SNP was also related to a decreased risk for BC in 5,649 cancer patients and 6,856 healthy controls. These results are inconsistent with our findings; this may be due to the limited sampling or low penetrance role in susceptibility to BC. The results of studies by Carrillo-Moreno on the ESR1 SNPs and BC in the Mexican population (17) and Sakoda LC on the ESR1 and androgen receptor SNPs in relation to BC risk and fibrocystic breast conditions among Chinese women (18) showed that the rs2234693 SNP is not a risk factor for BC.
Briefly, our study was done on 63 patients with BC and 65 healthy controls. We studied whether the rs1801132 and rs2234693 SNPs of the ESR1 gene are associated with susceptibility to BC. We found a significant association of the rs2234693 SNP (CC genotype) among patients with BC. Nevertheless, in this case-control study we did not find any significant correlation between the rs1801132 SNP with BC. In this study, a comparison of the BC patients versus healthy individuals showed a significant association between the CC genotype of the rs2234693 SNP and BC, whereas there was no significant correlation between the rs1801132 SNP and BC. As a result, it can be suggested that the rs2234693 SNP be considered for susceptibility to BC.