1. Background
Schistosomiasis, after malaria, is the second most important parasitic disease in the world in terms of morbidity and mortality and is one of the neglected tropical diseases, with approximately 90% of cases occurring in African regions (1). Because these diseases are more prevalent in certain areas and are scarce in some other areas, they are called by this name, which multiplies the epidemiological importance of these diseases and their early diagnosis (2). Six species of Trematoda cause schistosomiasis, including Schistosoma guineensis, S. haematobium, S. intercalatum, S. japonicum, S. mansoni, and S. mekongi. The disease appears in two primary forms: Urinary tract diseases caused by S. haematobium and intestinal diseases mainly caused by S. mansoni. Other species are much less common in morbidity and clinical symptoms. Schistosomiasis is transmitted through contact with fresh water contaminated by the human infection that contains the parasite eggs. The host snail should be present in the water in order for the parasite to complete the life cycle and infect the human (3).
Schistosoma haematobium is mainly found in tropical and subtropical areas with poor socioeconomic status and poor public health. Although it is distributed worldwide, it is more prevalent and distributed in the African region. It is estimated that at least 91.4% of people who need treatment for schistosomiasis live in Africa (4). The disease distribution depends on the intermediate host, i.e., Bulinus truncates snails from the truncatus group species (5). It is found in 76 countries and is endemic in 52 countries, mainly in developing countries in Asia, Africa, South America, and the Middle East, where almost 90% of the population needs treatment (6). In 2019, 236 million people were infected and suffered from schistosomiasis (7). At least 800 million people are at risk of being infected by this disease, leading to about 280,000 deaths each year (6). Recent reports indicate that in 2016, about 206.4 million people needed chemoprophylaxis treatment, while more than 89 million people were treated. Schistosomiasis control is implemented through periodic treatment, a large population treated with Praziquantel (PZQ), and a comprehensive approach to reducing disease transmission by accessing safe water, improving sanitation, hygiene education, and snail control. The World Health Organization (WHO) goal was to treat at least 75% of school children with PZQ in all endemic countries by 2020 (7).
The target groups for treatment include school children, adults at risk, and those who have jobs related to polluted water in endemic areas, such as fishermen, farmers, irrigation workers, and women exposed to contaminated water through domestic tasks. The treatment may be repeated several times each year in areas with high transmission. Monitoring is essential to determine the impact of control interventions. Schistosomiasis control in the past 40 years has been successful in several countries, including Brazil, Cambodia, China, Egypt, Mauritius, the Islamic Republic of Iran, Oman, Jordan, and Saudi Arabia. Some evidence shows that schistosomiasis has stopped in Morocco (8).
The disease has existed in Iran since 2,500 years ago. Based on field epidemiological studies, only seven spots in the Khuzestan province of Iran are endemic that are classified in terms of risk level into three regions: First-degree regions (rural areas of Dezful, Shush, Shushtar, Andimeshk, and Gotvand), second-degree regions (rural areas of the west of Ahwaz city and Azadegan plain) and third-degree regions (Khorramshahr, Abadan, Shadegan, and suburban villages) (9). The present study evaluated the endemicity variation of S. haematobium, including disease incidence and spatial-temporal clusters in the endemic regions of northern Khuzestan province in Iran from 1977 to 2001.
2. Methods
2.1. Study Plan and Data Collection
This cross-sectional study collected data from January 1977 to December 2001. The data of 1,390 confirmed cases with S. haematobium in the studied area, which were diagnosed and registered according to standard indicators, were investigated. The information included the province name, city name, rural district name, age, sex, type of treatment received, and the incidence time of illness. The disease incidence per 100,000 people in rural regions of the study area was evaluated over five-year periods, and the changes in the median age and sex of the patients were evaluated during this period.
2.2. Study Area
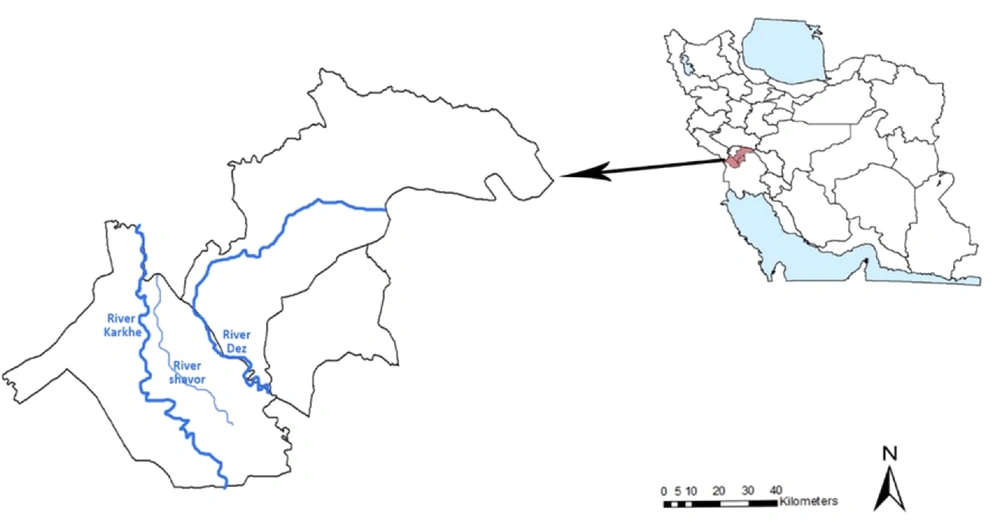
This study was carried out in the north of Khuzestan province (32°13’29.604 N, 48°29’22.833E), southwestern Iran, in three cities, including Dezful, Shush, and Gotvand. It has a semi-arid climate and an average temperature of 22.8°C (maximum temperature in the warmest month 53°C and minimum temperature in the coldest month 4°C), annual precipitation of 447.9 mm, and humidity of 44.5%. The maximum distance is approximately 128 km from east to west and 98 km from north to south. The region’s population studied was 78,415 people based on the latest census in 2016, and its total area is 9586 km2. This area has three permanent rivers, including the Dez River, Shavoor, and Karkheh (Figure 1).
2.3. Spatial, Temporal, and Spatiotemporal Cluster Analysis
Kulldorff’s retrospective space-time scan statistics (10) were used for the spatial and spatial-temporal distribution clustering of schistosomiasis using the SaTScan 8.0 software (Kulldorff and Information Management Services, Inc., Boston, MA, USA). Spatial scan statistic was used to detect and evaluate the clusters of schistosomiasis cases. It was done by gradually scanning a circle window across space and noting the number of observed and expected observations inside the window at each setting. The software performed the pure retrospective temporal analysis and retrospective spatial-temporal analysis to detect the temporal clusters of schistosomiasis cases with high rates using the discrete Poisson model. In this study, the time aggregation length was set to one month, and the maximum temporal cluster size was set to the default value of 50% within the study period.
SaTScan imposes circular windows of varying sizes on spatial data to detect the high-risk spatial clusters of cases. The window’s scanning radius was set at 50% in both at-risk populations and study periods in this study. Monte Carlo hypothesis testing (11) was used in this study, and the number of replications was set to 999 at a 0.05 significance level. The method identifies the most significant cluster and some secondary potential clusters.
3. Results
3.1. Schistosomiasis Incidence and Distribution According to Age and Sex
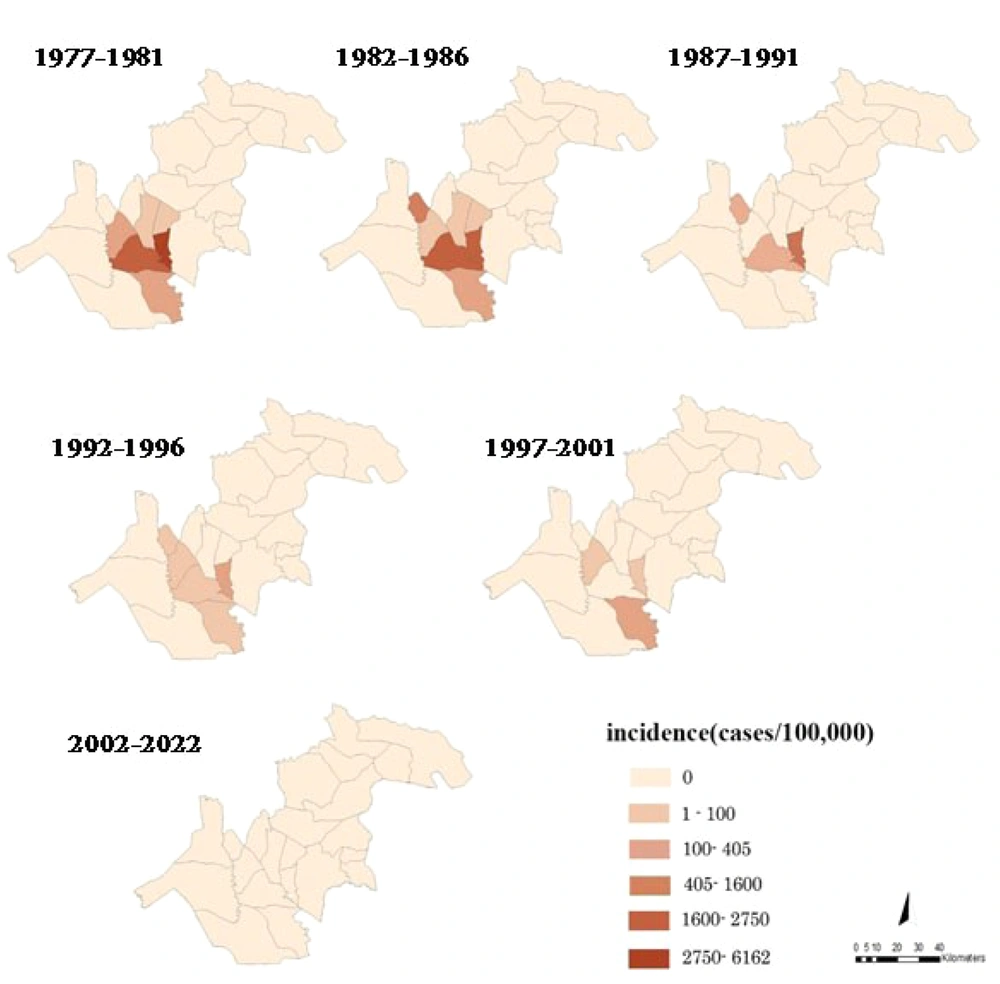
In the north of Khuzestan province, 1,390 patients with S. haematobium were reported from 1977 to 2001. The number of cases had a decreasing trend; the last case was reported in 2001, and no case was reported until 2022. Figure 2 shows the schistosomiasis incidence in rural districts over five-year periods. The incidence had a decreasing trend and declined from 256.8 per 100,000 in 1977 - 1981 to 1.6 per 100,000 in 1997 - 2001.
Table 1 shows that the median age at the time of diagnosis changed from 12 years at the beginning of the study period to 26.5 years in the end, showing an increasing trend. Moreover, the highest proportion in sex changed from 53.6% in males (423 cases) in 1977 - 1981 to 40% in males (four cases) in 1997 - 2001.
| Factors | Year | ||||
|---|---|---|---|---|---|
| 1977 - 1981 | 1982 - 1986 | 1687 - 1991 | 1992 - 1996 | 1997 - 2001 | |
| N a | 790 | 527 | 37 | 26 | 10 |
| Incidence rate (1/100000) | 256.8 | 169.1 | 7.9 | 5.5 | 1.6 |
| Median age | 12 | 10 | 12 | 21.5 | 26.5 |
| Sex | |||||
| Male | 423 | 294 | 23 | 11 | 4 |
| Female | 367 | 233 | 14 | 15 | 6 |
a N, the number of Schistosoma haematobium; the percentage values are in parentheses.
3.2. Cluster Distribution of Schistosomiasis
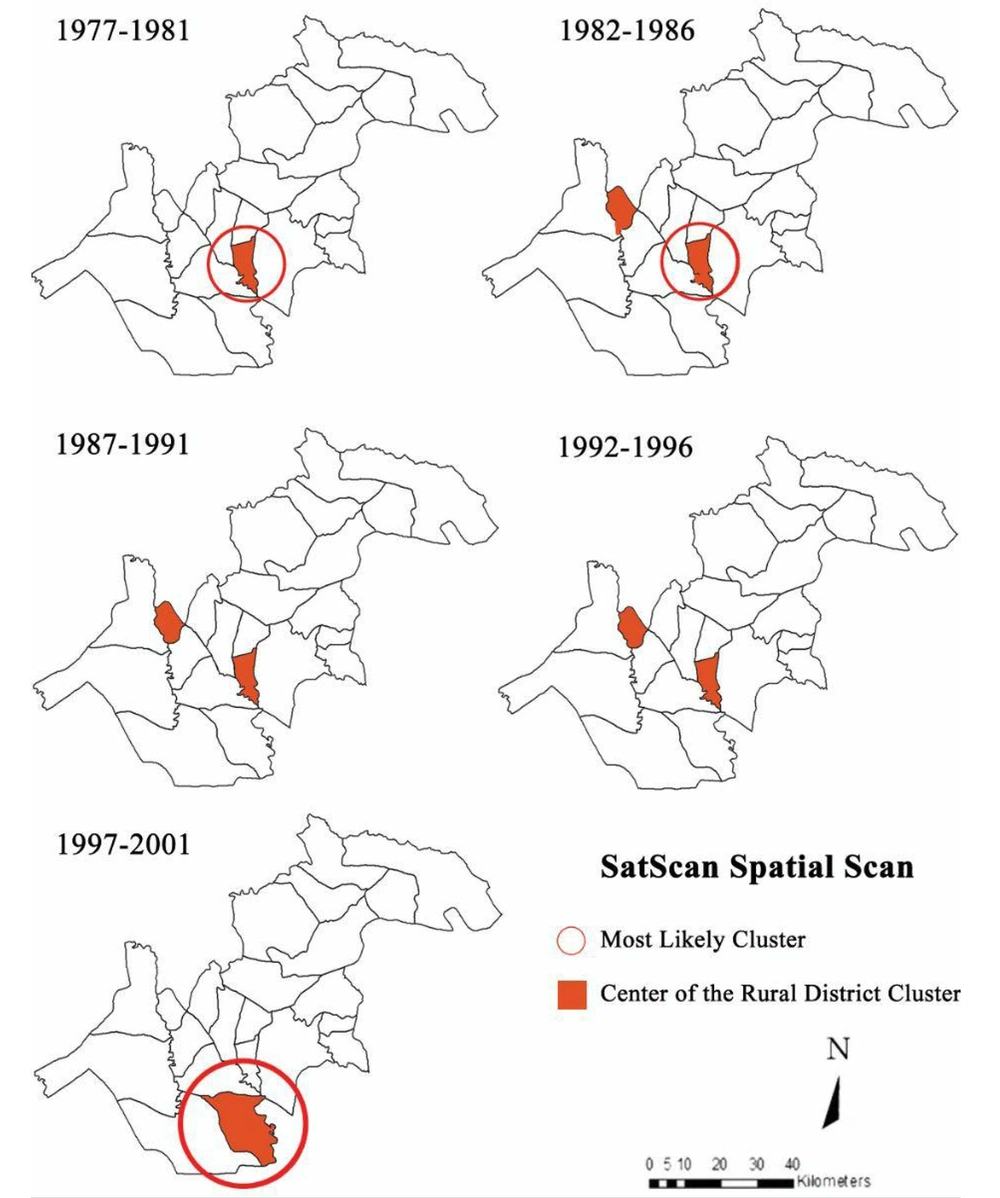
Kulldorff’s spatial scan method determined nine clusters in the north of Khuzestan province from 1977 to 2001 (Figure 3). In the first five-year period (1977 - 1981), one cluster is seen and in each of the following periods (1982 - 86, 1987 - 91, 1992 - 96, and 1997 - 2001), two clusters are seen. In the last five-year period (1997 - 2001), the two clusters overlap and are shown in the figure as a colored part.
Schistosoma haematobium had no random distribution in this region. The highest probability of the cluster was in Kheybar and Shavoor (LLR = 1777.40), and the lowest was in the Ben Moala rural district (LLR = 9.90) (P < 0.001).
3.3. Temporal Cluster Distribution of Schistosomiasis
Figure 3 shows temporal clusters. The temporal clusters of schistosomiasis cases in the north of Khuzestan province were 17 months and 5 days from 3/27/1973 to 3/19/1980 (629 cases), 11 days from 2/24/1982 to 3/7/1982 (93 cases), one day on 1/21/1987 (five cases), one day on 7/7/1993 (five cases), and one day on 1/2/1997 (three cases).
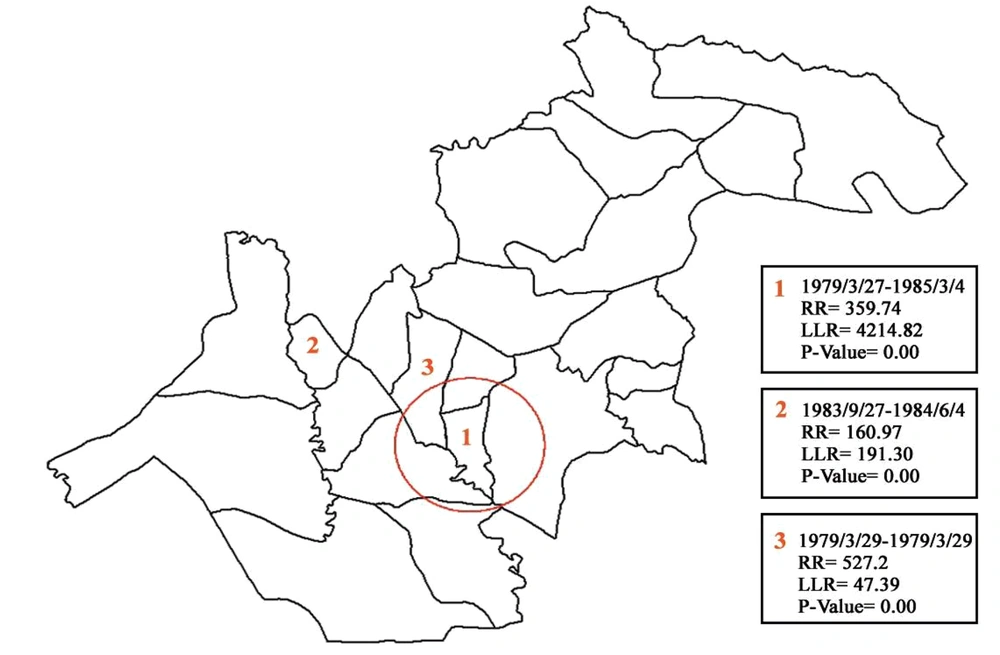
3.4. Spatial-Temporal Cluster Distribution of Schistosomiasis
Figure 4 shows the spatial-temporal cluster of schistosomiasis. Three clusters were determined between 1977 and 2001. These clusters included (1) Kheybar and Shavoor rural districts with the highest probability (LLR = 4214.82), (2) Ben Moala rural district (LLR = 191.30), and (3) Shamsabad rural district with the lowest probability (LLR = 47.39) (P < 0.0001).
4. Discussion
Khuzestan province, southwest of Iran, is considered the only endemic area of S. haematobium in Iran, and this disease has a long history in this area (12, 13). This study evaluated the incidence rate and endemic clusters of schistosomiasis in the north of Khuzestan province from 1977 to 2001, using spatial-temporal analysis at the rural district level. In this study, the median age of schistosomiasis patients changed from 12 years in 1977 - 1981 to 26.5 years in 1997 - 2001. According to this finding, the disease age increased in parallel with the decrease in disease incidence in the region.
In this study, the patients’ age increased over time and along with the decrease in the number of S. haematobium cases. More cases were observed in adults since, in the elimination stage, the only risk factor is accidental skin contact with water, which is accidentally infected naturally or with the patient’s urine from other endemic areas. Age, sex, and occupation also increase the chance of transmitting the parasite to humans and the spread rate. Therefore, increasing diagnosis age over time may be due to more adult exposure to infected water on farmland. On the other hand, open water resources are improved over time by improving dirt roads and turning them into concrete walls, decreasing children’s contact with water (9).
Previous studies from some endemic countries for schistosomiasis found that children aged 6 to 13 years were more infected with the disease (14, 15) due to more contact with contaminated water during swimming, bathing, and playing in open waters. Considering the study conducted on children in 1953, the infection rate in northern cities of Khuzestan province was 72% in the region from the Miyaneab area (near Ahvaz) to the north of Shush city, 2% in the Bidrubeh-ye region 40 km away from Andimeshk, and 10% in the eastern bloc in the south of Dezful, located between the Dez river on the western side and a branch of this river on the eastern side (9). The present study showed that the infection incidence decreased from 256.8 per 100,000 (790 cases) in 1977 - 1981 to 1.6 per 100,000 (10 cases) in 1997 - 2001. Despite the active surveillance in the health system, the disease has not been reported since 2001.
A significant reduction in the disease incidence and control was shown during the study period. These findings are consistent with the study that investigated 1.3 million urine samples from 1975 to 2013 in the infected sites of Khuzestan province in terms of S. haematobium eggs; the last positive case was reported in 2001 (13, 16). Our findings showed that the disease was higher in males (53.6%) between 1977 and 1981, consistent with studies conducted in Nigeria, Brazil, and Yemen (17-19). In 1997 - 2001, the disease was more prevalent in women (60% of the cases), consistent with the findings of studies in Ghana (20); this could be due to reducing the contact of men with contaminated sources in the investigated area. The transmission of schistosomiasis is affected by many key factors, such as climate conditions, the spatial distribution of the intermediate host (snail), and human activities (21). Even though schistosomiasis infection and its transmission in nature have a specific spatial heterogeneity, it can be determined by the scale of the community, the region, or even smaller scales of a rural area (22-24).
In this study, Kulldorff’s spatial scan method identified different clusters of schistosomiasis in the north of Khuzestan province in the rural district area (10). The results showed nine clusters in the study period, and the spatial distribution of S. haematobium was characterized by a pattern in which clusters and the regions with high incidence were located along the Dez and Shavoor rivers. This finding is similar to the results of some previous studies, which reported the relationship between Schistosoma infection and the proximity of the river and accessible water sources (24-26). Furthermore, there are also large sugar cane fields in these areas that increase the risk of contact with the source of infection. As shown in some studies, the risk of urinary schistosomiasis increases with issues related to agriculture (27).
All nine clusters observed during the study period in the north of Khuzestan province vanished in 2002, and the endemic condition of the disease ended. However, there is still active surveillance, such as monitoring and investigating the snail vector habitat (9). Ending the endemic condition can be attributed to the successful implementation of the national program and several projects for controlling schistosomiasis in contaminated sites, including population training, environmental improvements (such as the massive and free distribution of building materials and bowls for upgrading toilets), reducing contact with contaminated water, such as swimming, fishing, diving, and optimizing wastewater treatment systems, extensive drug treatment of infected populations by PZQ, and controlling snails (16, 28).
The pure temporal cluster analysis showed the high-risk months of schistosomiasis for each period from 1977 to 2001. The temporal clusters decreased from 17 months and 5 days in 1977 - 1981 to one day in 1997 - 2001, and then temporal clusters vanished. The clusters in the north of Khuzestan province were more frequent in the winter and summer, possibly due to the favorable weather conditions in winter, increasing the proliferation and density of snail vectors in this region. Some laboratory studies show that B. truncatus snails have the most extended lifetime in winter (12). Also, due to the warm weather and the increasing desire of people to swim in Dez and Shavoor rivers and other open water sources in summer, the probability of contact with the source of contamination increases. The geographical distribution of schistosomiasis is proportional to the presence and density of freshwater snails, which is essential for their presence as an intermediate host (29). The snail species is B. truncatus in Khuzestan province (30, 31). Changes in climatic conditions, such as the increasing temperature of the area, reducing rainfall, changes in traditional systems to agricultural and industrial systems, changes in crop cultivation patterns, such as reducing or stopping the area under sugarcane cultivation, improving open water transfer routes to arable lands and subsequently drought, and the control measures of snails can play a significant role in reducing the density of snails in the north of Khuzestan province. In previous studies, B. truncatus was positive in seven regions of Khuzestan province, and the prevalence was 6% (32, 33).
In 2019, a definite S. haematobium case was observed in a 25-year-old man with military occupation. The patient was admitted to an army hospital and was identified in Tehran city, a non-endemic area for the disease. This person was probably infected due to a trip to Iraq (34). Although no disease has been identified or reported in the study area since 2002, there is a possibility of infection due to carrier snails, local people traveling to Iraq, living people of Iraq in Khuzestan province, and swimming and washing habits in rivers. Therefore, the care system for S. haematobium is still needed.
We acknowledge some limitations in the present research, which require further discussion. First, the spatial distribution of schistosomiasis was analyzed in the northern rural districts of Khuzestan province, which may lead to ecological bias. Plotting the spatial patterns of schistosomiasis at the rural surface could lead to more accurate results (24). Second, our study analyzed only the cases reported by the healthcare system, which may not reflect the actual number of patients and may lead to low reporting; however, it was the best and most accurate source for accessing retrospective data. Third, schistosomiasis data were retrieved from archived documents, so there was the possibility of losing some data and, consequently, information bias. Also, using systematic review articles may not be an excellent alternative to unpublished articles (35). Finally, there was a limitation in analyzing other variables based on the information recorded in the documents.
4.1. Conclusions
The findings indicate a zero incidence and the disappearance of S. haematobium endemicity in the northern region of Khuzestan province of Iran in 2002. Due to snail vectors of the disease resident in this region, monitoring the disease is still necessary. According to the latest WHO report (2018), countries successful in controlling the morbidity and transmission chain of schistosomiasis are classified as low-transmission countries, and they should attempt the next phase, i.e., disease elimination. It is worthwhile that the obtained results are documented based on guidelines. The transfer of experiences from other high-risk areas can provide influential achievements in controlling the disease and join the prosperous countries in cutting the disease transmission chain.