1. Background
Heart failure (HF) is a clinical, multifactorial syndrome complicated by etiology, in which dysfunction of the heart occurs, such as a defect in the pumping of blood to other parts of the body (1, 2). It is now well known that this disease causes hemodynamic, metabolic, and neurohumoral changes and causes clinical symptoms such as shortness of breath, muscle fatigue, swelling of the lower extremities, decreased cardiac output, and decreased tissue blood flow. Pulmonary congestion (2, 3) According to the World Health Organization, the disease is a significant threat to the health of many communities. Today more than 23 million people worldwide are infected with the disease annually (4, 5). On the other hand, numerous clinical evaluations show that failure Cardiac is one of the most common causes of hospitalization and mortality in the elderly the prevalence of this disease in men is almost twice as high as in women (6). Cardiovascular diseases (CVDs) are the most important causes of mortality worldwide, and it is estimated that the overall number of deaths caused by CVDs will be increased to 20 million by 2030 (7, 8). Despite numerous epidemiological and etiological studies in this field, the etiology of this disease is still in a state of ambiguity, and despite extensive advances in medicine, the prognosis of HF syndrome remains poor (9). However, studies show that HF is a multifactorial syndrome and can be closely related to underlying disorders such as hypertension, diabetes mellitus, kidney disease, and anemia (10, 11). Therefore, in recent years, anemia has been mentioned as an essential independent factor in the hospitalization and death of patients with HF syndrome (12-15). According to the World Health Organization, if the hemoglobin level in an adult male is below 13 mg/dL and the hemoglobin level in an adult female is below 12 mg/dL, there is anemia (15, 16). Numerous studies suggest that anemia can be considered a predictor of the course of HF (17), but what obscures the issue is that anemia is an integral feature of aging in many societies. It is for various reasons (18), and this can question the predictability of anemia as a prognosis for HF syndrome (19). The association between low hemoglobin (< 12 mg/dL) and hematocrit (< 34%) and the side effects of heart failure has long been confirmed in numerous studies (17) and what the literature shows is that A large number of patients with HF have anemia. But what is doubtful is whether the cause of HF is anemia or anemia is one of the causes of complications from HF syndrome (20).
2. Objectives
As mentioned earlier, aging is one of the causes of increased incidence of HF (21). On the other hand, anemia in old age is one of the essential clinical features of many diseases (22). Therefore, in this study, to answer this question, we seek to investigate HF syndrome according to the rate of anemia in the subjects and its relationship with mortality due to the severity of the disease to understand better provide the cause of HF and anemia and provide a solution to the prognosis of HF and anemia.
3. Methods
3.1. Ethics Statement
All procedures were performed according to the ethical guidelines of the Faculty of Medical Sciences, Dezful University of Medical Sciences. The study adhered to the principles of the Declaration of Helsinki. All study participants received a full explanation of the study and signed written informed consent before their inclusion in the study.
3.2. Selection of People
This case-control study was performed on patients who were referred to Ganjavian Hospital in Dezful (southeast Iran). A sample of male and female elderly with the considered characteristics were selected. For the purpose of the study, an elderly was defined as a person between 50 and 70 years old. In this study, 273 people over 50 years old were selected from 700 patients with HF admitted to the CCU and POST CCU wards of Ganjavian Hospital (Dezful General Hospital, Khuzestan Province, Iran). All study participants if they had inclusion criteria, including HF, non-smoking, and no exclusion criteria, such as having any disorders other than cardiovascular failure, such as the history of surgery, neurological disorders, urological disorders, and lack of genetic diseases related to blood, and also the age above and below the age range considered in this study were selected for the study. The cardiologist diagnosed the ejection fraction and confirmed that they had HF. The time of diagnosis of infected people was between 2012 and 2014, and the age limit of all participants in this study was between 50 and 70 years old due to the study on the elderly. It should be noted that the subjects in this study received all the usual medications related to their HF, and there was no change in the participants' treatment regimen. We used the World Health Organization (WHO) definition of anemia in adults (males < 13 g/dL and females < 12 g/dL) in our study (23).
3.3. Sample Preparation
Venous blood samples were collected in EDTA tubes and then centrifuged for 30 minutes. The obtained plasma was frozen and stored at -80°C until analysis. The maximum shelf life was two months and did not exceed the time recommended by the manufacturer. Blood sampling was performed on the days of cardiac examination after fasting for 8 - 12, and 9 cc of blood was taken from each person. In the first stage, biochemical parameters were measured in the standard medical diagnostic laboratory of Dezful Hospital. Each serum sample was excluded from the study due to hemolysis, and sampling was repeated for the patient in the following days or was excluded from the study if the patient was not available.
3.4. Laboratory Methods
The weight and height of participants with light clothing and without shoes were measured, and body weight index or BMI was calculated as the ratio of weight (kg) to height square (m2). Fasting blood glucose (FBS) was measured on the serum of patients by GOD / PAP enzymatic method using a laboratory kit made by Pars Azmoun Company (Iran). Plasma triglyceride (TG) and total cholesterol (TC) levels, as well as plasma minerals, such as Ca, were measured according to the protocol kits of Pars Azmoun Company (Iran) and routine laboratory methods. Serum potassium and sodium were measured using an ISE device (Germany). BT 3000 autoanalyzer was used to determine the level of serum creatinine and blood urea nitrogen (BUN). Hematological tests, such as hematocrit, hemoglobin, RBC (red blood cell), WBC (white blood cell), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC) were measured using the CBC Sysmex hematologic autoanalyzer. Finally, the type of drugs used, comorbidities, and length of hospital stay due to HF were recorded by a researcher-made questionnaire.
It should be noted that all participants in this study read and signed the consent form to enter this study. All laboratory methods were performed according to the ethical instructions prepared by the Medical Ethics Committee of Dezful University of Medical Sciences.
3.5. Statistical Analysis
The results were statistically analyzed. After examining the normality of data by SPSS software version 16, the difference between people with HF and normal people in terms of demographic, biochemical, and coagulation parameters was evaluated by an independent t-test and presented as mean ± SEM. Then, the Pearson correlation coefficient was calculated for hematological and biochemical measurements with the values of cardiac tests in people with HF. The significance level was considered as P < 0.05 for all statistical results.
4. Results
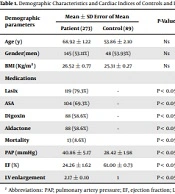
Table 1 presents the results of demographic characteristics and cardiac indices of controls and patients.
| Demographic Parameters | Mean ± SD Error of Mean | P-Value | |
|---|---|---|---|
| Patient (273) | Control (89) | ||
| Age (y) | 68.92 ± 1.22 | 53.86 ± 2.10 | Ns |
| Gender(men) | 145 (53.11%) | 48 (53.93%) | Ns |
| BMI (Kg/m2) | 26.52 ± 0.77 | 25.31 ± 0.27 | Ns |
| Medications | |||
| Lasix | 119 (79.3%) | - | < 0.05 |
| ASA | 104 (69.3%) | - | < 0.05 |
| Digoxin | 88 (58.6%) | - | < 0.05 |
| Aldactone | 88 (58.6%) | - | < 0.05 |
| Mortality | 13 (8.6%) | < 0.05 | |
| PAP (mmHg) | 40.86 ± 5.17 | 28.42 ± 1.98 | < 0.05 |
| EF (%) | 24.26 ± 1.62 | 61.00 ± 0.73 | < 0.05 |
| LV enlargement | 2.17 ± 0.10 | 1 | < 0.05 |
Demographic Characteristics and Cardiac Indices of Controls and Patients
As shown in Table 1, there was no significant difference in age, sex, and BMI between the control group and patients. But in terms of drug therapy, mortality rate, and cardiac indices (PAP, EF, and LV), there was a significant difference between the patients and controls. It should be noted that no changes were made in the routine treatments of the patients according to the ethical protocol of Dezful University of Medical Sciences during this study, and digoxin and aldactone (58.6%) were used. In addition, the results showed that 13 patients (8.6%) died during hospitalization and these people had the lowest hemoglobin level.
The biochemical findings of the participants in this study are presented in Table 2.
| Biochemical Parameters | Mean ± Std. Error of Mean | P-Value | |
|---|---|---|---|
| Patient (273) | Control (89) | ||
| FBS (mg/dL) | 136.63 ± 5.56 | 89.36 ± 2.45 | < 0.05 |
| TG (mg/dL) | 109.26 ± 4.95 | 113.73 ± 8.94 | NS |
| TC | 172.56 ± 7.02 | 135.22 ± 4.87 | < 0.05 |
| HDL | 48.31 ± 1.29 | 72.16 ± 3.53 | < 0.05 |
| LDL | 96.42 ± 3.30 | 80.30 ± 6.03 | NS |
| VLDL | 22.36 ± 1.10 | 26.90 ± 2.41 | NS |
| Na | 138.32 ± 0.83 | 140.40 ± 0.61 | NS |
| K | 4.20 ± 0.06 | 4.25 ± 0.09 | NS |
Comparison of Data Obtained from Measuring Biochemical Parameters Between Patients and Controls
As shown in Table 2, patients with HF had significantly higher FBS and TC than the control group in this study. Patients had significantly lower HDL than the control group. But in terms of other biochemical indicators, no significant difference was observed between the controls and patients.
| Hematologic Parameters | Mean ± Std. Error of Mean | 2-tailed P-Value | |
|---|---|---|---|
| Patient (273) | Control (89) | ||
| Hematocrit (%) | 38.36 ± 0.59 | 41.53 ± 0.61 | 0.02 |
| Percentage of anemic person (%) a | 62 | 8 | 0.016 |
| Hemoglobin (g/dL) | 11.94 ± 0.15 | 14.80 ± 0.28 | 0.4 |
| MCV (fl) | 87.63 ± 0.81 | 89.53 ± 0.92 | 0.18 |
| MCH (pg) | 27.93 ± 0.23 | 28.93 ± 0.30 | 0.01 |
| WBC × 1000 | 9.62 ± 0.31 | 6.63 ± 0.34 | 0.004 |
| RBC × 1000000 | 4.65 ± 0.17 | 5.14 ± 0.22 | 0.039 |
| Plt × 1000 | 236.25 ± 10.6 | 315.83 ± 12.87 | 0.62 |
| RDW | 14.87 ± 0.30 | 13.40 ± 0.18 | 0.01 |
Comparison of Data Obtained from the Measurement of Hematological Parameters Between Patients and Controls
A comparison of hematological data in the patients and control group (Table 3) showed that hematocrit, hemoglobin, RBC, and MCH levels in the group of patients with HF were considerably lower than in the control group and in contrast, WBC and red blood cell distribution width (RDW) in patients group were significantly more than the control group. There was no significant difference in platelet count and MCV between the controls and patients.
Table 4 shows the Pearson correlation coefficient of important blood parameters in terms of anemia, namely HCT, HB, RBC, and MCV, with cardiac parameters studied in this study, including ejection fraction (EF), and pulmonary artery pressure (PAP), and left ventricular wall elongation (LV enlargement) in patients with HF.
| HCT | Hg | RBC | MCV | |
|---|---|---|---|---|
| EF | -0.201 (0.01) | -0.109 (0.18) | -0.133 (0.10) | 0.030 (0.72) |
| LV | 0.383 (0.008) | 0.206 (0.20) | 0.121 (0.41) | -0.067 (0.65) |
| PAP | -0.293 (0.289) | 0.531 (0.04) | 0.476 (0.07) | -0.472 (0.08) |
Pearson Correlation Coefficient of Hematological Parameters and Cardiac Parameters Among Patients with Heart Failure a
As shown in Table 4, there was a significant correlation between the amount of ER and the amount of left ventricular wall elongation with the amount of hematocrit, i.e., the percentage of hematocrit change causes negative and positive changes in EF and LV parameters, but has an effect on pulmonary artery pressure. On the other hand, the number of RBCs and hemoglobin levels were significantly correlated with the amount of pulmonary artery pressure, which means that these parameters affect each other. Also, mean MCV showed a significant correlation with PAP levels in people with HF.
5. Discussion
Numerous studies have investigated the role of anemia in the development of disorders, such as HF (24). In the present study, this role and its incidence were investigated in patients with HF who were referred to the Cardiovascular Diseases Center of Dezful Hospital. According to the World Health Organization (WHO), adult men with hemoglobin below 13 mg/dL and adult women with hemoglobin levels below 12 mg/dL have anemia (25). Here, we analyzed the presence of anemia in a group of elderly patients, including patients with cardiovascular disease. We found that patients with heart disease were more likely to have anemia. More than 50% of patients enrolled at the time of hospitalization were anemic. Based on the results of biochemical, hematological, and cardiac studies of patients with HF in the present study, it was determined (Tables 1-3) that anemia criteria based on WHO criteria in studied patients were more than the control group, which may worsen the prognosis for HF in people with HF. Our results in confirmation of other studies showed that anemia and an increase in its severity could increase the incidence of more secondary serious diseases caused by HF and lead to a poor prognosis and increased mortality rate due to HF (26-28). In a similar study by Spazzafumo et al. (29), they found a negative correlation between Hb and CRP, suggesting that anemia may be a marker associated with the inflammatory process in the elderly. In addition, recent studies strongly suggest that aging is related to the deregulation of proinflammatory cytokines, particularly interleukin-6, which may adversely affect hematopoiesis, either by inhibiting erythropoietin (EPO) production or by interacting with EPO receptors (30, 31) In a study in line with our study by Morici et al., older patients with CVD and anemia in the last 24 months had a higher risk of death than other people with anemia (31, 32). Although few studies have shown that anemia, determined at a single point in time, is associated with a worse prognosis in MI and/or HF patients, only a few studies have examined the effect of anemia in HF patients (32, 33). Here, we showed that the risk of death in anemic patients with HF was almost twice that of non-anemic patients. Our results are consistent with a previous report, which suggests that anemia in HF patients is associated with an increased risk of congestive heart failure (CHF) hospitalization, major bleeding, and mortality from all causes (34). This result was obtained by including only HF patients with chronic HF under occluded artery trial (OAT) in survival analysis to avoid possible bias due to different effects on drug mortality (35). There is still unknown whether HF is the cause of anemia or anemia is the cause of HF (36). Two arguments may be made about the cause and effect of anemia for HF. The first argument is that in our study, the age of the subjects under study for HF was between 50 and 70 years, and today it has been found that the prevalence of anemia increases with age (37). Many studies have found that the cause of anemia in old age is often background disorders, such as iron deficiency, and chronic diseases in old age, such as gastrointestinal bleeding (38).
On the other hand, several studies have shown that with age, the risk of cardiovascular diseases, including HF, increases (39), and this anemia may be the initiator of HF in old age (40). However, it is now known that people with congenital HF in the early stages of their disease do not have anemia according to WHO criteria. With the development of HF, anemia will be observed in them (41). Aging, in addition to anemia caused by HF, has been imposed on the patient, and this is probably why we are witnessing more disorders due to HF at older ages (42, 43). Several studies have shown that HF can be a multifactorial and sometimes complex disorder (44, 45), and the role of genetic and hereditary factors in the etiology of HF has been confirmed today (46), and as mentioned earlier, there are patients with HF, whose disorder can only be justified by genetic and congenital pattern and anemia cannot be considered a cause for their disorder (47, 48). Also, in our study, it was found that increasing the severity of anemia is likely to worsen the HF in patients and improve their mortality rate (Table 3). Our findings, in line with the results of other studies, showed that the mortality rate is significantly higher in people with HF who have more severe anemia than in people with HF with milder anemia, and often HF is more observed in people with more severe anemia (49, 50).
Our findings simply showed that HF might be the starting point for the early onset of anemia, especially in middle age and old age, and still continued anemia due to aging is likely to exacerbate HF. Therefore, in addition to being a prognostic factor for HF, anemia can be one of the most critical factors in controlling HF from the cardiologists’ viewpoint, and this is the case for older people who are at higher risk for anemia. In addition, the results of this study showed that aging could be regarded as one of the threats of increased anemia and HF (51), and possibly in the elderly with HF, anemia should be considered more diligently in treatment lines.
5.1. Conclusions
It can be concluded that HF and anemia in our studied area, as in other studies in other parts of the world, are closely related, and HF may be the cause of anemia, and it is less likely that anemia is a cause of HF. However, the increase in the severity of anemia may have increased the threat of HF in patients. Perhaps one of the essential treatment strategies to control secondary disorders caused by HF is the control of anemia. Our findings simply showed that HF might be the starting point for early onset of anemia, especially in middle age and old age, but continued anemia due to aging is likely to exacerbate HF.