1. Background
Headache is a common complaint in the general population, particularly in females. It is a purely mental experience that produces undesirable hemodynamic and metabolic responses for women (1). Primary headaches (when pain is the disease), such as migraine, tension, and nervous headache, account for most headaches in young women (2).
Postpartum headache occurs in about 30 - 40% of all women in the first week after delivery, with tension headaches first followed by migraine headaches (3, 4). Studies have shown that tension and migraine headaches are much more common in pregnant women compared to the other types of headaches (5). Secondary headaches (such as dural puncture in spinal anesthesia) are quite typical. It is a positional character, which occurs by a decrease in cerebrospinal fluid (CSF) pressure within 1 to 7 days after dural puncture (2).
Cesarean section is one of the most common gynecological surgeries (6), which has become more common in recent years due to numerous factors (7, 8). Spinal anesthesia is the most commonly chosen method of anesthesia for cesarean section, which is a popular technique due to its simplicity and high reliability, as well as the speed of achieving adequate anesthesia (9). This method has fewer complications for mother and baby than general anesthesia and reduces the intensity of postoperative surgical pain (10); however, postdural puncture headache (PDPH) is one of the most common complications following spinal anesthesia, which is caused by rupture of the dura, CSF leakage, and meningeal traction (11). Seventy percent of PDPH go away within 7 days (12). PDPH is the sixth most common cause of postpartum headache. After cesarean section, the most important thing is to diagnose different types of headaches. Many mothers experience a postpartum headache that has nothing to do with spinal anesthesia (3, 4).
The diagnosis of PDPH and its treatment have always been considered by anesthesiologists (13). PDPH occurs by changing posture from lying to sitting and sitting to standing. PDPH sometimes has a series of symptoms and signs such as nausea, vomiting, neck pain, and shoulder pain (14). PDPH causes adverse psychological responses in the mother, including anxiety, sadness, aggression, insomnia, and failure to develop the relationship between mother and baby, as well as a reduction in milk supply and the mother’s tolerance for breastfeeding. PDPH properties are often helpful in its diagnosis from other causes of postpartum headache. The presence of tension headache is the major risk factor accompanied by the incidence of PDPH in women undergoing cesarean delivery (15).
PDPH causes an increased incidence of postpartum depression, posttraumatic stress disorder, chronic headache and backache, and decreased breastfeeding following PDPH. It emphasizes the follow-up of postpartum headache and distinguishes PDPH from other types of headache in the postpartum period (16).
Previous PDPH history indicates a higher chance of a new episode of PDPH after spinal anesthesia. These women are more susceptible to such recurrences (17). Patients with a history of chronic headaches were more likely to have PDPH. The type of chronic headache did not affect the incidence of PDPH (18). Women’s psychological status affects the development of PDPH regardless of preexisting headache or previous PDPH (19).
2. Objectives
The aim of this study was to identify the effects of 2 needle types on PDPH in elective cesarean surgery. The first parameter was the incidence of PDPH in 2 groups. Then, the mean severity of the headache and some related factors (such as movement limitation, day’s number of PDPH, and need to take analgesic) were followed in each group among PDPH women for 1 week after surgery. Also, associated symptoms and signs (including nausea, vomiting, neck and shoulder pain) were compared in the 2 groups for 1 week after surgery.
3. Methods
This study was a randomized prospective trial. After obtaining the code of the ethics committee, we selected women candidates for elective cesarean section based on inclusion and exclusion criteria. Then, we obtained informed consent from all of them. All women were in ASA I physical status. The research environment was the university hospitals of Rafsanjan University of Medical Sciences in 2020.
Two groups of women underwent spinal block with 2 types of needles, Whitacre 25 gauge, and Quincke 25 gauge. The women in the 2 groups had the same conditions such as the amount of fluid therapy during fasting, level of anesthesia T4 procedure (paramedian), fixed 0.05% heavy Marcaine drug with volume constant 3 to 3.5 cc, constant speed of infusion in intrathecal space, sitting position, and intervertebral space L3-L4 or L4-L5.
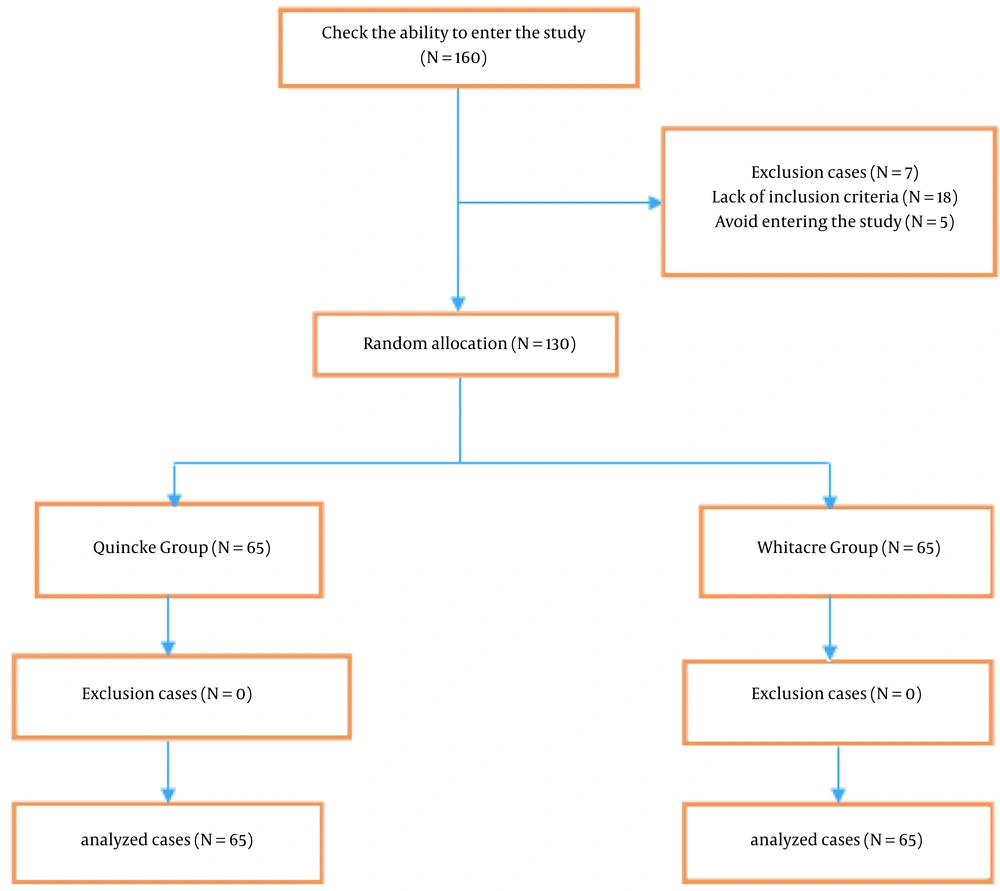
The sample size of this study was calculated with the level of confidence 0.95 and margin of error 0.5 (20). The 130 women candidates for elective cesarean section were randomly divided into 2 equal groups. To this purpose, using the excel file and random between function, 65 numbers were randomly selected from numbers 1 - 130 according to the number of women entering the study to assign to the Whitacre group, and the remaining numbers were assigned to the Quincke group. All women were blinded to the type of needle.
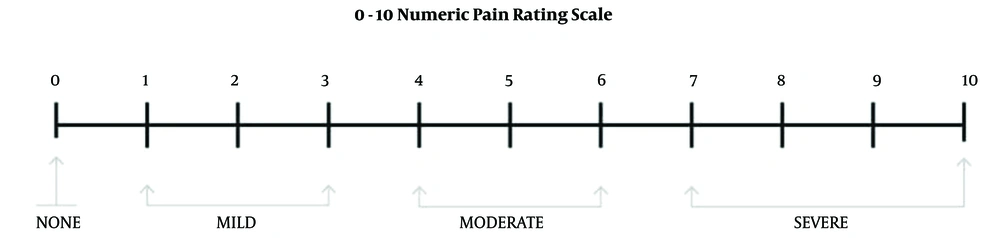
To conduct this study, a checklist was designed for each parturient within 1 week after cesarean. We considered the primary outcome PDPH in the checklist. In the case of PDPH, secondary outcomes (such as the severity of PDPH, days of PDPH in the parturient, need to analgesic, and movement limitation) were evaluated for 1 week after surgery. Also, associated symptoms and signs (such as neck pain, shoulder pain, sensitivity to sound and light, nausea, and vomiting) were evaluated for 1 week after surgery. The Visual Analogue Scale (VAS) measurement was used to measure the severity of PDPH. In this method, the women who suffered from PDPH were asked to place a mark on the vertical line to detect the personal experience of pain, as shown in Figure 1 (21).
Exclusion criteria included failure on the first try, failure to complete painless level need for cesarean, eclampsia, preeclampsia, refusal of spinal anesthesia by women, and women who entered the study but were not accessible to fill the checklist a week later.
The flow diagram of the studied women is reported in Figure 2.
The resulting data were analyzed using SPSS version 22 (SPSS Inc, Chicago, Ill, USA). The variables in this study include 2 categories: quantitative and qualitative variables. Quantitative variables include the mean severity of PDPH and day’s number of PDPH. Qualitative variables include the need for analgesia, movement limitation, and associated symptoms and signs, such as nausea, vomiting, and shoulder and neck pain. The quantitative variables were presented as mean ± SD, and the qualitative variables were presented as frequency and percentage. It should be noted that the studied indicators were compared only in the parturient with PDPH between the 2 groups.
The Kolmogorov-Smirnov test was used to evaluate the normality of the mean severity score of PDPH and the other quantitative indicators. The equality of variances in the groups was also evaluated by the Levene test. The t-test and related post-tests were used to compare the groups. In addition, to compare the incidence of PDPH and associated symptoms and signs in the 2 groups of parturients, the independent 2-sample t-test and Fisher exact test were used. A significant level of 5% (95% confidence interval) was considered.
4. Results
The results related to mean age, parity, body mass index (BMI), history of primary headaches (migraine, tension), and nervous headache (headache at operation time) were evaluated in the 2 groups and presented in Table 1.
| Variables | Whitacre Group | Quincke Group | P-Value |
|---|---|---|---|
| Age (y) | 30.15 ± 5.66 | 30.29 ± 5.48 | 0.909 |
| Parity | 2.14 ± 0.90 | 2.29 ± 1.13 | 0.11 |
| BMI (kg/m2) | 29.12 ± 4.02 | 30.11 ± 4.67 | 0.11 |
| History of primary headaches (yes) | 4 (6.1) | 1 (1.5) | 0.365 |
| Nervous headache (yes) | 2 (3) | 0 (0) | 0.496 |
a Values are expressed as mean ± SD or No. (%).
The total number and distribution of the severity of PDPH in the 2 groups are presented in Table 2. PDPH was developed following spinal anesthesia in 16 women (12.3%), including 6 women in the Whitacre group and 10 women in the Quincke group. Table 3 compares the measured indices after spinal block in the 2 groups for 1 week after surgery. The mean severity was more in the Whitacre group than in the Quincke group.
| Groups | PDPH Incidence | PDPH Severity | ||
|---|---|---|---|---|
| Severe | Moderate | Mild | ||
| Whitacre group | 6 (9.2) | 3 (50) | 2 (33.3) | 1 (16.6) |
| Quincke group | 10 (15.4) | 3 (30) | 2 (20) | 5 (50) |
a Values are expressed as No. (%).
| Variables | Whitacre Group | Quincke Group | P-Value |
|---|---|---|---|
| Mean severity of PDPH | 7.17 ± 2.40 | 4.80 ± 3.29 | 0.121 |
| Days of PDPH | 7.50 ± 3.20 | 4.40 ± 2.91 | 0.082 |
| Need for analgesia (yes) | 5 (83.3) | 5 (50) | 0.307 |
| Movement limitation (yes) | 4 (66.7) | 5 (50) | 0.633 |
| Associated symptoms and signs (yes) | 5 (83.3) | 6 (60) | 0.588 |
a Values are expressed as mean ± SD or No. (%).
The associated symptoms and signs in the Whitacre group were 83.3% (ie, neck pain in 1 woman, sound sensitivity in 2 women, shoulder pain in 1 woman, and severe nausea and sensitivity to sound in 1 woman), and in the Quincke group were 60% (ie, neck pain in 2 women, nausea in 1 woman, shortness of breath and chest pain in 1 woman, neck pain and sound sensitivity in 1 woman, and shoulder pain and sensitivity to sound and light in 1 woman).
5. Discussion
Since primary headaches (such as tension, migraine, and nervous headaches) are common complaints in young women, and PDPH is a secondary headache due to intentional dura puncture in spinal anesthesia, we evaluated the incidence of PDPH as the primary outcome in young women. The other parameters (such as the severity of PDPH, movement limitation, need to analgesic, days of PDPH, and associated symptoms and signs of PDPH) were considered secondary outcomes. We planned to study women who received spinal anesthesia for elective cesarean section. Accidentally, the Whitacre group had more history of primary headaches. Although the number of PDPH was less in the Whitacre group than in the Quincke group, it was not statistically significant. It can be related to more history of primary headaches in the Whitacre group that caused the number of PDPH women to be more in this group. Also, secondary outcomes (such as the mean severity of PDPH, movement limitation, need to analgesic, days of PDPH, and associated symptoms and signs) were more apparent in the Whitacre group than in the Quincke group. It can be due to more number of women with a history of primary headaches in the Whitacre group.
One of the reasons that most women who received spinal anesthesia do not present PDPH is that dura mater can attenuate or even prevent the CSF loss after a puncture through a dynamic phenomenon of orifice occlusion (22).
In a study in 2008, a previous history of PDPH indicated a higher chance of a new episode of PDPH after spinal anesthesia. Women were more susceptible to such recurrences (17). In another study in 2017, the major risk factors associated with the incidence of PDPH in women undergoing cesarean delivery were repeated puncture attempts and the presence of tension headache (15).
In a study, it was suggested that the presence of previous chronic headaches increased the risk of developing PDPH; also, in patients with PDPH and previous chronic headaches, there was a delayed clinical worsening of previous chronic headaches (18).
To date, no study has been conducted on the secondary outcome of PDPH and associated symptoms and signs of it in young women. In this study, the secondary outcomes of PDPH (such as mean severity, movement limitation, days of PDPH, need for analgesic, associated symptoms and signs [such as nausea, vomiting, shoulder pain, and neck pain], and sound and photosensitivity) were more apparent in the Whitacre group than in the Quincke group. Secondary outcomes and associated symptoms and signs are important because each of these complaints restricts women from performing housekeeping duties and caring for their child, as well as increases thromboembolic events in women, especially overweight women.
Our results should be interpreted in the context of the study’s limitations. Since PDPH was only followed for 7 days after spinal anesthesia, some women may have shown PDPH after 7 days, which was not evaluated in our study.
5.1. Conclusions
The results of this study showed that although the number of women with PDPH was more in the Quincke group, the difference was not statistically significant. This could be because of more women with a history of primary headaches in the Whitacre group than in the Quincke group. Also, secondary outcomes and associated symptoms and signs of PDPH were more apparent in the Whitacre group than in the Quincke group. This could be due to more history of primary headaches in the Whitacre group than in the Quincke group. However, there was no statistically significant difference in all of the PDPH indices between the 2 groups, which could be due to the low number of PDPH in the present study.