1. Background
Ischemic stroke patients may suffer from various complications due to immobilization, such as deep vein thrombosis (DVT), pressure ulcers, or sarcopenia, which may lead to bone fractures (1).
DVT is a serious complication in patients with acute ischemic stroke and can lead to severe pulmonary embolism, which may result in death (2). DVT prevalence in ischemic stroke patients ranges from 20 to 70%, and it may differ depending on the detecting method (3). Therefore, there should be a prophylactic treatment against DVT.
Khan et al. recommended a low-dose anticoagulant therapy for ischemic stroke (4).
Low molecular weight heparin (enoxaparin) is routinely chosen for stroke patients in whom hemorrhagic stroke has been excluded (1). According to the evidence, a dose of 30 mg twice daily is likely insufficient for prophylactic treatment in DVT patients (5). Optimal enoxaparin dosing for prophylactic treatment in DVT remains elusive. Dosing between 0.3 - 0.5 IU/mL could decrease the prevalence of DVT without increased complications (5). Currently, in the ASEAN population, particularly in Vietnam, there is a limited number of DVT prophylaxis studies in patients hospitalized for acute cerebral ischemia. Therefore, prophylaxis information, reference values, and complication rates are still limited. Meanwhile, early and appropriate DVT prophylaxis with the correct drug dose produces a successful treatment.
2. Objectives
This study aims to evaluate the benefits and risks of prophylactic treatment in DVT patients with dosing of enoxaparin (Lovenox of Sanofi-Aventis Vietnam Company) 40 mg (4,000 units of anti-Xa, 0.4 mL), once daily.
3. Methods
3.1. Study Design and Research Objects
This cross-sectional study is conducted on patients with acute ischemic stroke who were hospitalized at the Intensive Care and Toxicology Unit or the Internal Neurology Department of Ca Mau General Hospital, Vietnam, from June 2020 to July 2021.
The inclusion criteria were:
- Clinical criteria: Those older than 18 years old with focal neurological deficits persisting for over 24 hours, which are sudden onset, and no history of traumatic brain injury.
- Para-clinical criteria: CT scan results of the skull with cerebral injury in the form of cerebral infarction with reducing attenuation and lack of cerebral hemorrhage images.
- Criteria for selecting cerebral infarction: Patients whose disease was begun a week ago; Patients who have received basic treatment according to the American Heart Association's guidance for the early management of stroke in the acute.
The exclusion criteria included: (1) having evidence of DVT while screening or evidence of hemorrhage; (2) medical history or evidence of intracranial hemorrhage; (3) heparin-induced thrombocytopenia (HIT); (4) spinal or epidural anesthesia or lumbar puncture within 24 hours; (5) treating thrombolysis within 24 hours; (6) coma during screening; (7) presence or suspicion of the aneurysm or cerebrovascular malformation; (8) malignancy increases the risk of bleeding or interferes with monitoring or evaluation of outcomes; (9) decreased hemostasis, such as platelet count < 100,000/μL, a PTT greater than 1.5 times of the upper limit of normal, or INR > 1.5; (10) allergy or history of allergy to heparin.
3.2. Sample Size
The sample size was estimated as 58 subjects, based on the DVT prevalence in ischemic stroke patients (3) with a 95% confidence interval, α = 0.05, d = 0.09, and P = 0.131, using the following formula:
3.3. Research Content
General characteristics of the study include clinical examination, clinical indicators evaluation, paraclinical test, and diagnostic imaging. Clinical examination included age, blood pressure, gender, and risk factors. Clinical indicators evaluation included body mass index (BMI), the International Medical Prevention Registry on Venous Thromboembolism Scale (IMPROVE Scale), Glasgow Coma Score, The National Institutes of Health Stroke Scale (NIHSS stroke Scale), and immobilization standards. Para-clinical tests included complete blood count (Sysmex Xn–1000 Celldyn Ruby, America), coagulation function tests (Sysmex CS-2000i STA Compact MAX, America), and serum biochemistry (Beckman Coulter AU-680, America). Diagnostic imaging included cranial CT-scan (Philips CT scanner), electrocardiogram (NIHON KOHDEN (9620 L-2000), Japan), and Doppler ultrasound (Samsung SONOACE R7, Korea).
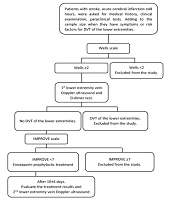
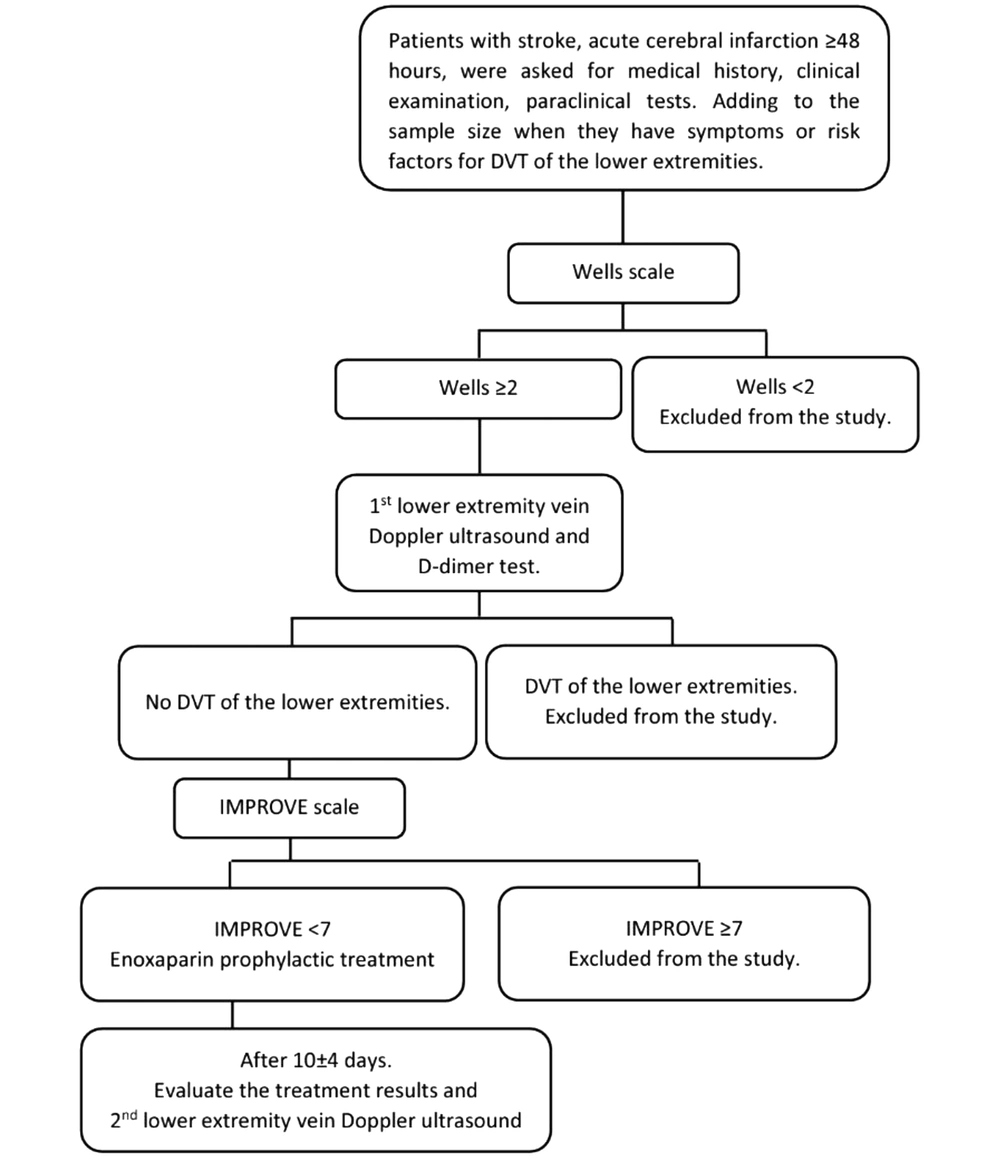
The risk of DVT in the lower extremities was evaluated according to the Wells Scale (6). Prophylactic treatment of the lower extremities DVT was performed according to a unified regimen of the Vietnam National Heart Association (7) with low molecular weight heparin (Lovenox of Sanofi-Aventis Vietnam Company); dosage: 40 mg (4,000 units of anti-Xa, 0.4 mL) once a day; administration: subcutaneous injection once a day; usage duration: median 7 days of prophylactic treatment (based on risks assessed by Wells Scale) (Figure 1). Evaluation of treatment outcomes included re-evaluation of the Glasgow Coma Scale, NIHSS, 2nd Doppler ultrasound of the lower extremities with the press test, and complete blood count (Figure 1). Complications while using enoxaparin included hemorrhage (cerebral hemorrhage, gastrointestinal bleeding, bladder bleeding, intra-abdominal bleeding, lower bleeding skin, nosebleeds, ...) and heparin-induced thrombocytopenia (HIT) (8). General outcomes of the treatment were categorized as stable, unstable, and death.
3.4. Analysis and Processing of Data
Data obtained from medical records were entered into a computer and analyzed using SPSS version 20.0. Qualitative variables were described by frequency or rate, while continuous quantitative variables were described by the mean (standard deviation). Quantitative and discrete variables were described using relative and absolute frequencies. Inter-group comparisons were performed using the χ2 (chi-square) test and student t-test. Univariate and multivariable logistic regression models were applied to identify related factors. Statistical significance was considered when the P-value < 0.05, with a 95% confidence interval.
3.5. Research Ethics
Our research strictly adhered to ethical criteria in medical research and was approved by the Scientific Council of Can Tho University of Medicine and Pharmacy and Ca Mau General Hospital (code: No.30/HDDD-PCT on 27th May 2020).
4. Results
4.1. General Characteristics of the Study
General characteristics of participants (through quantitative variables) are provided in Table 1. The mean age of participants was 73.55 ± 11.67 (year), while the mean value of BMI was 21.15 ± 5.2 (kg/m2), and immobilization standards were 4.48 ± 1.56 (days). The mean value of the Wells Scale was 2.14 ± 0.35, and IMPROVE Scale was 2.98 ± 1.4. Table 2 showed the general characteristics of the patient through qualitative variables. Of all participants, 51.7% were male. The prevalence of D-dimer positive patients (D-dimer > 0.55 mg/L) was 81%. Smoking was the most prevalent risk factor (43.1%).
| Characteristic | Mean, |
|---|---|
| Clinical examination | |
| Age (y) | 73.55 ± 11.67 (32 - 99) |
| Systolic blood pressure (mmHg) | 139.14 ± 10.3 (90 - 200) |
| Diastolic blood pressure (mmHg) | 79.66 ± 6.2 (50 - 100) |
| Clinical indicators evaluation | |
| BMI (kg/m2) | 21.15 ± 5.2 (16.6 - 29.4) |
| IMPROVE Scale | 2.98 ± 1.4 (0 - 6.5) |
| Glasgow Coma Score | 11.43 ± 2.57 (7 - 15) |
| NIHSS Stroke Scale | 18.07 ± 6.1 (7 - 33) |
| Wells Scale | 2.14 ± 0.35 (2 - 3) |
| Immobilization standards | 4.48 ± 1.56 (2 - 7) |
| Paraclinical test | |
| Hemoglobin (g/dL) | 12.2 ± 1.54 |
| Hematocrit (%) | 37.5 ± 4.31 |
| Platelet Count (k/µL) | 258.3 ± 89.44 |
| PT (s) | 11.2 ± 1.19 |
| aPTT (s) | 26.4 ± 3.06 |
| INR | 1.06 ± 0.11 |
| Fibrinogen (g/L) | 4.60 ± 1.52 |
| Glucose (mg/dL) | 130.8 ± 39.04 |
| D-dimer (mg/L) | 3.45 ± 6.41 |
| Cholesterol (mg/dL) | 146.5 ± 45.54 |
| Triglycerid (mg/dL) | 120.5 ± 70.51 |
| HDL-c (mg/dL) | 34.6 ± 9.32 |
| LDL-c (mg/dL) | 99.8 ± 36.46 |
| Characteristics | Rate; No. (%) |
|---|---|
| Gender (male) | 30 (51.7) |
| Risk factors | |
| Family history DVT | 5 (8.6) |
| Diabetes mellitus | 12 (20.7) |
| Smoking | 25 (43.1) |
| Hospitalization in the last two months | 14 (24.1) |
| Heart failure | 8 (13.8) |
| Dyslipidemia | 7 (12.1) |
| COPD or asthma | 5 (8.6) |
| Using contraceptive pills | 0 |
| D-dimer > 0.55 (mg/L) | 47 (81) |
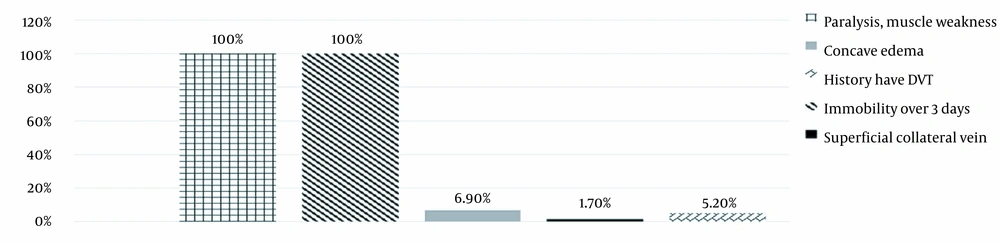
Figure 2 showed that 100% of cases in the study had paralysis, muscle weakness, and immobility over 3 days. There were 4 cases (6.9%) of concave edema in the leg. Also, one case (1.7%) had superficial collateral vein. Three cases had (5.2%) DVT.
4.2. Enoxaparin Prophylactic Treatment Result
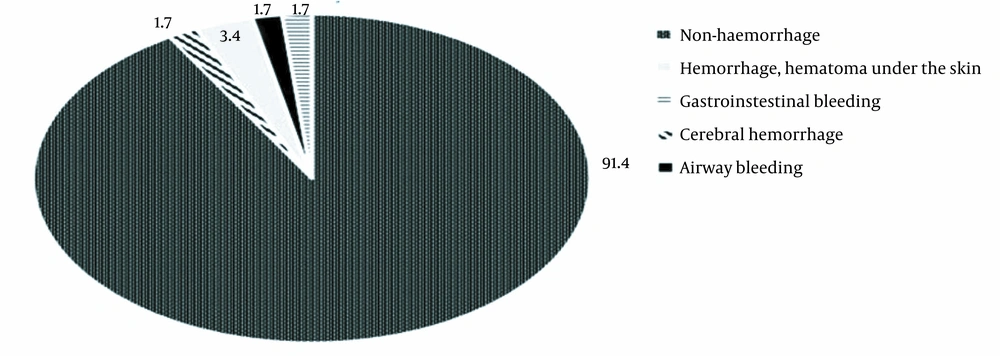
Following deep vein prevention with enoxaparin, no case had DVT in a lower extremity. According to Figure 3, two cases (3.4%) had hemorrhage and hematoma under the skin at the injection site ≥ 5 cm. Also, one case (1.7) experienced airway bleeding, and gastrointestinal bleeding was observed in one case (1.7%). As shown in Table 3, the difference in mean scores of Glasgow and NIHSS before and after treatment was 0.76 (CI: 0.55 to 0.96) and 1.07 (CI: 0.69 to 1.44), respectively. This difference was statistically significant (Glasgow: t57 = 7.41; P < 0.001, NIHSS: t57 = 5.66; P < 0.001). As shown in Table 4, there was one case of thrombocytopenia > 50% after providing the intervention. Noteworthy, we did not observe any bleeding complications. There were 5 cases of bleeding, ranging from mild to severe, in the group of < 30% thrombocytopenia. According to Table 5, the difference in mean platelet counts before and after treatment was -13.29 (CI: -33.89 to 7.3), which was not statistically significant (t57 = -1.29; P = 0.20).
| Variables | Before Treatment ( | After 10 ± 4 Days of Treatment ( |
|---|---|---|
| Glasgow | 11.43 ± 2.57 | 12.19 ± 2.30 |
| NIHSS | 18.07 ± 6.11 | 17.00 ± 6.15 |
| Complications | Percentage of Platelets Decreased After Treatment with Enoxaparin; No. (%) | Total | ||
|---|---|---|---|---|
| < 30% | 30 - 50% | > 50% | ||
| None | 50 (86.2) | 2 (3.4) | 1 (1.7) | 53 |
| Cerebral hemorrhage | 1 (1.7) | 0 | 0 | 1 |
| Gastrointestinal bleeding | 1 (1.7) | 0 | 0 | 1 |
| Hemorrhage, hematoma at the injection site | 2 (3.4) | 0 | 0 | 2 |
| Airway bleeding | 1 (1.7) | 0 | 0 | 1 |
| Total | 55 | 2 | 1 | 58 |
| Variable | Before treatment ( | After 10 ± 4 days of treatment ( |
|---|---|---|
| Platelet count 103/µL | 258.26 ± 89.44 | 271.55 ± 79.99 |
5. Discussion
5.1. General Characteristics of Participants
A total of 58 patients with acute ischemic stroke were investigated. The mean age of participants was 73.55 ± 11.67 years (Table 1). Lawall et al. studied 102 ICU patients in Germany and reported that the mean age of men and women was 71.4 ± 11.4 and 75.9 ± 14.0, respectively (9). In our study, 51.7% (n = 30) of participants were male (Table 2), which is similar to the study by Phuoc (53.9% male and 46.1% female). Thao noted that 75.1% of their participants were male, and 24.9% were female (10, 11). Also, in the study by Miri et al., 58.18% of participants were male (12). In this study, the mean value of BMI was 21.15 ± 2.26 (Table 1), almost similar to the study by Thao. The mean value of BMI of the sample was 20.7 ± 2.3, which is consistent with the common mean value of BMI of hospitalized patients in Vietnam (10).
The meantime of immobilization to hospitalization to enoxaparin prophylaxis was 4.48 days. According to the Wells score, all patients were immobilized with a risk of 1 point (Figure 2). Immobilization can reduce the flow of blood vessels, especially at the venous valves, leading to inflammation and hypercoagulability.
In our study, 81% of D-dimer was positive (> 0.55 mg/L). Thao reported that 45.48% of patients were positive for D-dimer, including 29.2% of patients with lower extremities DVT. There was no difference between patients with positive and negative D-dimer (10). In a longitudinal study on 304 acute medical patients, Phuoc found that D-dimer concentration in the group of patients without DVT was 589 ng/mL. In the group of patients with DVT, the mean concentration of D-dimer was 816 ng/mL (P < 0.001). The cut-off value of D-dimer to diagnose DVT in the group of at-risk patients was 500 ng/mL with a sensitivity of 77.8%, a specificity of 42.8%. DVT was completely excluded when the concentration of D-dimer was below the diagnostic threshold of 500 ng/mL (11). Therefore, the D-dimer test is not specific for patients who need intensive resuscitation and have DVT, which can also be considered an acute medical disease (13).
In our study, smoking was the most common risk factor, followed by prolonged hospital stay. Smoking increases plasma fibrinogen levels and causes blood cells to become more sticky than normal. This makes it easier for blood clots to form. According to Thao, there is a statistically significant association between smoking and DVT (OR = 2.88; 95% CI: 1.7 - 4.7; P < 0.001) (10). Heit et al. and White concluded that approximately 20% of DVT events progressed during the hospital stay or within 2 months of being hospitalized for more than 4 days. DVT still recurs frequently during the first few months after the initial event, with a recurrence rate of about 7% at 6 months. Nearly 6% of DVT patients lose their lives and the mortality rate of pulmonary embolus within 1 month of diagnosis is 12% (14, 15).
5.2. Enoxaparin Prophylactic Treatment Result
Concerning the prevention of lower extremities DVT by enoxaparin (40 mg per day), the results indicated 100% prevention (i.e., zero incidence). Thao, in a study that used a dose similar to our study, reported that the rate of newly diagnosed DVT in the prevention group was 13.4% and in the non-prophylaxis group was 43.7%; this difference was statistically significant (P < 0.001) (10). Wang et al. also found a significant decrease in DVT risk in prophylaxis with low-molecular-weight heparin (pooled OR 0.56, 95% CI 0.41 to 0.77, P < 0.001) (16). According to Western studies, the incidence of DVT after prophylaxis was 5.5% for MEDENOX study (17), 2.8% for PREVENT study (18), and 5.6% for ARTEMIS study (19). These differences may be due to differences in patient populations, co-morbidities, study duration, and methods of diagnosing DVT. All studies showed that the rate of new DVT in the prevention group was lower than in the non-prophylaxis group; the difference was statistically significant.
In our study, there was one case (1.7%) of a cerebral hemorrhage after day 4 (Figure 3). Two cases (3.4%) had hemorrhage and hematoma under the skin at the injection site ≥ 5 cm. Also, one case (1.7) experienced airway bleeding, and gastrointestinal bleeding was observed in one case (1.7%) (Figure 3). The results of this study are similar to studies conducted in the West that reported a low rate of heavy bleeding. For instance, Samama et al.: 1.7% and 1.1% (20), Fraisse et al.: 5, 6% and 2.7% (21), Lazoroviz et al.: 0.5% and 0.2% (18), and Cohen et al.: 0.2% and 0.2% (19). A systematic review by Wang et al. also reported similar results; so that there was a significant increase in postoperative hemorrhage in patients treated with enoxaparin than those who did not receive anticoagulation treatment (pooled OR 1.87, 95% CI 1.05 to 3.31, P = 0.033) (16). They reported no significant difference in the rate of severe bleeding between the prevention and non-prophylaxis groups (P > 0.05).
We also investigated thrombocytopenia on admission time. There was one case (1.7%) of thrombocytopenia > 50% after providing the intervention, but there was no case with bleeding complications (Table 4). In the group of < 30% thrombocytopenia, five cases presented mild to severe bleeding (Table 4). The difference in mean platelet counts before and after treatment was -13.29 (CI: -33.89 to 7.3) (Table 5), which was not statistically significant (t57 = -1.29; P = 0.20). Thao reported six cases (1.7%) of thrombocytopenia: 2 cases (1.3%) in the prevention group and 4 cases (1.9%) in the non-prevention group (10). There was no statistically significant difference between the prophylaxis and non-prophylaxis groups concerning the rate of thrombocytopenia (P = 0.651) (10). The rate of thrombocytopenia in the study was similar to that of prevention studies conducted abroad. Samama et al. (20), in a prevention study, reported rates of 2.2% and 3.6%, and Lazoroviz et al. (18) reported rates of 0.3% and 0.5%. These studies reported no statistically significant difference in the incidence of thrombocytopenia between the prevention and non-prophylaxis groups. Enoxaparin was not the primary cause, and thrombocytopenia was only an incidental symptom in patients with and without prophylaxis. Therefore, thrombocytopenia > 50% is not the main cause of major bleeding complications.
5.3. Limitations and Implementations
It is necessary to mention some limitations of our study, including the relatively short evaluation period, which was due to the short hospitalization of patients and low incidence of DVT. Long periods of staying in bed for immobilized patients may lead to an increased incidence of DVT. We propose a multicenter study with more participants and a longer patient evaluation period. With good prophylactic results, early prophylactic treatment of lower extremity DVT with enoxaparin is recommended in at-risk patients. DVT prevention with enoxaparin should be promoted for patients with acute cerebral infarction at General Hospitals.
5.4. Conclusions
This study demonstrated that enoxaparin is not the primary cause of thrombocytopenia. The prevention of DVT with enoxaparin in patients with an acute ischemic stroke presented a good effect. This intervention is quite safe, so that it does not increase the risk of severe bleeding and does not increase the risk of thrombocytopenia.