Dear Editor,
As of March 25, 2022, a total of 7,145,877 confirmed cases of coronavirus disease 2019 (COVID-19) and 139,865 COVID-19-related deaths were reported in Iran. Public vaccination against COVID-19 started on March 2021. The first group who received the first vaccine doses were health care professionals. Before the era of vaccination, public lockdown, wearing masks, and using viral disinfectants were the major protection methods against the circulating severe acute respiratory syndrome coronavirus 2 (SARS-Cov-2). The vaccination against COVID-19 in Iran started with vaccines produced in non-western countries. Sputnik V (developed by Gamaleya) and Covilo (developed by Sinopharm) were among the first vaccine types administered in Iran. Later, locally produced vaccines were also applied to expedite the process of vaccination against the pandemic and lower the mortality rate.
So far, there have been several strategies for developing vaccine against SARS-CoV-2. The common feature of all these platforms is to teach the human immune system to orchestrate a fast and efficient immune response against the invading virus. Basically, COVID-19 vaccines are categorized into two groups; first, vaccines based on immunogenic components of the SARS-CoV-2, and second, whole virus vaccines.
Vaccines based on immunogenic components of the SARS-CoV-2 are based on five different mechanisms, including protein subunit, virus-like particles (VLPs), DNA-based or RNA-based vaccines, non-replicated viral vectors, and replicating viral vectors. Protein subunit-based vaccines are SARS-CoV-2-isolated and purified proteins, which show immunogenic properties and trigger humoral immune responses. VLP-based vaccines are produced from viral proteins that mimic the structure of the real SARS-CoV-2. However, these types of vaccines do not contain any genetic material. DNA-based or RNA-based vaccines are vehicles for the introduction of viral genetic material (which may be DNA or mRNA). This approach leads to efficient production of viral proteins in the recipient leading to immune reactions against the produced viral proteins. Non-replicated viral vector-based vaccines contain genetic material to produce immunogenic viral proteins packaged inside an artificial risk-free virus not proficient for self-replication. Moreover, replicating viral vector-based vaccines is similar to non-replicated viral vector-based vaccines in terms of construct and functionality except for the replication capability.
Whole virus vaccines for SARS-CoV-2 are either “inactivated viruses” or “live-attenuated viruses”. Inactivated virus-based vaccines contain a large number of SARS-CoV-2, which have been killed using various safe methods. live-attenuated virus-based SARS-CoV-2 vaccines contain a large number of SARS-CoV-2, which have been weakened.
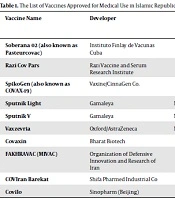
As of November 19, 2021, Iran imported 148,784,308 doses of COVID-19 vaccines, which included 131,498,898 doses of the Sinopharm BIBP vaccine from China, 7,850,600 doses of the Oxford-AstraZeneca vaccine from various countries (including China, South Korea, Italy, Netherlands, Austria, Poland, Russia, and Greece), 4,091,200 doses of the Sputnik V vaccine from Russia, and 1,125,000 doses of the Bharat Covaxin from India (1). Alongside these vaccines, several vaccines developed in Iran were also added to the list of available vaccines for vaccination against COVID-19. Of note, Pasteurcovac and COVAX-19 are two locally produced SARS-CoV-2 vaccines in Iran produced in collaboration with other countries. Pasteurcovac has been developed in collaboration with Cuba, and COVAX-19 has been developed in collaboration with an Australian company. Table 1 shows the list of COVID-19 vaccines, name of their funder and developer, vaccine types and action mechanism, number of countries the vaccine has been approved in, and the number of countries the vaccine clinical trials have been conducted in. Moreover, Table 2 summarizes a list of locally developed COVID-19 vaccines in Iran with their approval statues, approval date, and their different clinical trials in Iran. It is worth mentioning that Pasteurcovac does not have any clinical trials (finished or ongoing) in Iran.
| Vaccine Name | Developer | Vaccine Type | Number of Countries Approved in | Number of Countries with Clinical Trials in (Clinical Trials) |
|---|---|---|---|---|
| Soberana 02 (also known as Pasteurcovac) | Instituto Finlay de Vacunas Cuba | Protein subunit | 4 | 1 (3 clinical trials) |
| Razi Cov Pars | Razi Vaccine and Serum Research Institute | Protein subunit | 1 | 1 (5 clinical trials) |
| SpikoGen (also known as COVAX-19) | Vaxine/CinnaGen Co. | Protein subunit | 1 | 2 (7 clinical trials) |
| Sputnik Light | Gamaleya | Non replicating viral vector | 26 | 2 (6 clinical trials) |
| Sputnik V | Gamaleya | Non replicating viral vector | 74 | 7 (24 clinical trials) |
| Vaxzevria | Oxford/AstraZeneca | Non replicating viral vector | 138 | 30 (61 clinical trials) |
| Covaxin | Bharat Biotech | Inactivated virus | 14 | 2 (10 clinical trials) |
| FAKHRAVAC (MIVAC) | Organization of Defensive Innovation and Research of Iran | Inactivated virus | 1 | 1 (3 clinical trials) |
| COVIran Barekat | Shifa Pharmed Industrial Co | Inactivated virus | 1 | 1 (6 clinical trials) |
| Covilo | Sinopharm (Beijing) | Inactivated virus | 90 | 11 (24 clinical trials) |
The List of Vaccines Approved for Medical Use in Islamic Republic of Iran
| Vaccine Name | Developer | Approval Statues (Approval Date) | Phase I Clinical Trial ID (Number of Patients) | Phase II Clinical Trial ID (Number of Patients) | Phase III Clinical Trial ID (Number of Patients) |
|---|---|---|---|---|---|
| Razi Cov Pars | Razi Vaccine and Serum Research Institute | Approved (31 October 2021) | IRCT20201214049709N1 (133) | IRCT20201214049709N5 (210) | IRCT20201214049709N2 (500) |
| IRCT20201214049709N2 (500) | |||||
| SpikoGen | Vaxine/CinnaGen Co. | Approved (6 October 2021) | - | IRCT20150303021315N23 (400) | IRCT20150303021315N26 (300) |
| IRCT20150303021315N24 (16,876) | |||||
| FAKHRAVAC (MIVAC) | Organization of Defensive Innovation and Research of Iran | Approved (9 September 2021) | IRCT20210206050259N1 (135) | IRCT20201214049709N2 (500) | IRCT20201214049709N2 (500) |
| COVIran Barekat | Shifa Pharmed Industrial Co | Approved (13 June 2021) | IRCT20201202049567N2 (32) | IRCT20171122037571N3 (500) | IRCT20201202049567N3 b (20,000) |
| IRCT20201202049567N1 (56) | |||||
| Noora | Bagheiat-allah University of Medical Sciences | Not approved | IRCT20210620051639N1 (70) | IRCT20210620051639N2 (300) | IRCT20210620051639N3 (10,300) |
| OSVID-19 | Osve Pharmaceutical Company | Not approved | IRCT20210622051670N1 (40) | - | - |
The List of Locally Produced COVID-19 Vaccines in Iran with Their Approval Statues and Different Phases of Clinical Trials in Iran a
In conclusion, it is safe to claim that vaccines are the best and most efficient approach for training our immune system to recognize, fight, and eliminate invading viruses. In this approach, our immune system produces neutralizing antibodies against viral particles essential for the cellular internalization and replication of the invading virus. Therefore, the immune system creates a defensive wall against the virus and efficiently protects the host. When a vaccinated individual is re-exposed to SARS-CoV-2, the immune system quickly recognizes the viral antigens and mediates immune response against them.
To bring this pandemic to an end and have the lowest COVID-19-related mortality, a large share of the population needs to be fully vaccinated. Receiving three doses of a vaccine type helps prepare the immune system for future viral exposures. According to statistics, as of March 25, 2022, about 67% of the Iranian people have been fully vaccinated. Furthermore, 8.4% of the people have been partly vaccinated. Therefore, as of the mentioned date, 75% of the people have been vaccinated at least for one dose (2, 3). Conclusively, we recommend vaccination as the most effective way of protection against this life-threatening infectious disease, as vaccination has been the most important step in controlling various pandemics during the past century. However, some researchers proposed that the protection mediated by the vaccines are temporary and only last for a few months.