1. Background
Multiple sclerosis (MS) is one of the most common debilitating neurological diseases in young adults, often between the ages of 20 and 40 (1). It manifests as variable symptoms such as optic neuritis, fatigue, spasticity, neuro-urological dysfunction, paresthesia, and headache (2). Weather, sunlight, and day/night dynamics were studied as possible causes of MS (1). The accepted risk factor involved in this geographic trend is low levels of vitamin D. Exposure to sunlight by increasing vitamin D levels protects against MS (3). Temperature and light intensity daily fluctuations and changes during the day and temperature in various seasons are the key elements in maintaining the human circadian rhythm (4). The circadian clock system regulates the physiological, metabolic, and behavioral daily rhythms in mammals. The molecular mechanisms of the circadian rhythm system include several circadian genes, and the circadian locomotor output cycles kaput (CLOCK) as one of these genes plays a vital role in a wide range of behaviors (5). The circadian clock system includes several negative feedback loops of transcription-translation, as well as post-transcription and post-translational modifications in circadian proteins (6, 7). Its key function depends on transcription activation of major downstream genes and increasing chromatin opening, as well as regulating DNA access to other transcription factors (8, 9).
The CLOCK gene in the chr4q12 position with 28 exons and the aryl hydrocarbon receptor nuclear translocator-like (ARNTL) gene with 25 exons are among the most important genes in the internal clock system. To maintain circadian rhythms, the CLOCK protein can form a heterodimer with BMAL1 (encoded by the aryl hydrocarbon receptor nucleus transmitter (ARNTL), which attaches to the E-box promoter and activates the transcription of the period and cryptochrome genes. The PER and CRY proteins are heterodimerized and complexes in the cytoplasm. They migrate to the nucleus and inhibit CLOCK and BMAL1 activity (10, 11). Simultaneous expression of circadian genes is essential for organizing the 24-hour cycle (6). Research revealed working shifts at a young age raises the development risk of MS (12). In addition to sleep deprivation/restriction, shift work disturbs circadian cycles, leading to dysfunction of melatonin secretion and increased pro-inflammatory activity, and can contribute to inflammatory reactions in MS development. Melatonin is involved in the circadian and seasonal rhythms directly (13) and directly affects the CLOCK and ARNTL expression through post-transcription and/or translation activities (14). There is a rising suggestion that molecular clocks and time of day affect immune function. Shift workers, frequent air passengers, and populations more prone to circadian disturbances increase the incidence of chronic inflammatory disease (15). Discoordination of circadian rhythms is associated with various disorders such as neurodegenerative diseases, cardiovascular, cancer, and also immune system dysfunction (16, 17). However, disruption in the circadian rhythms leads to dysregulation of the immune system and may be related to increased risk for MS (12, 17). Therefore, the aim of this study was to determine genetic changes of the ARNTL and CLOCK genes as circadian rhythm-regulating genes in the positions rs3789327 and rs6811520 that might increase the MS risk.
2. Objectives
This study aimed at genotyping rs3789327 in the ARNTL and rs6811520 in the CLOCK genes and their association with MS in the Iranian population.
3. Methods
3.1. Ethics Statement
The study was approved by the ethical committee of the Dezful University of Medical Sciences (IR.DUMS.REC.1400.037). This study is based on the Helsinki Declaration. All patients and the control group signed an informed consent form.
3.2. Patients
The present study was carried out on 100 patients diagnosed with relapsing-remitting multiple sclerosis (RRMS) who were referred to the MS clinic of the Ayatollah Kashani hospital in Isfahan, Iran. Also, 100 healthy controls referred to the Dezful blood transfusion center were selected as the control group that matched based on age and sex with patients. MS patients were definitively diagnosed, according to MacDonald criteria (18).
3.3. Genotyping
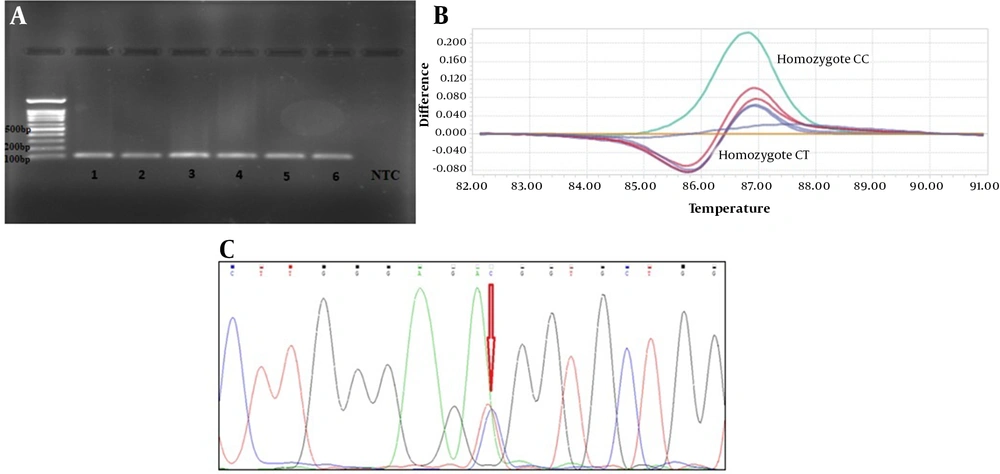
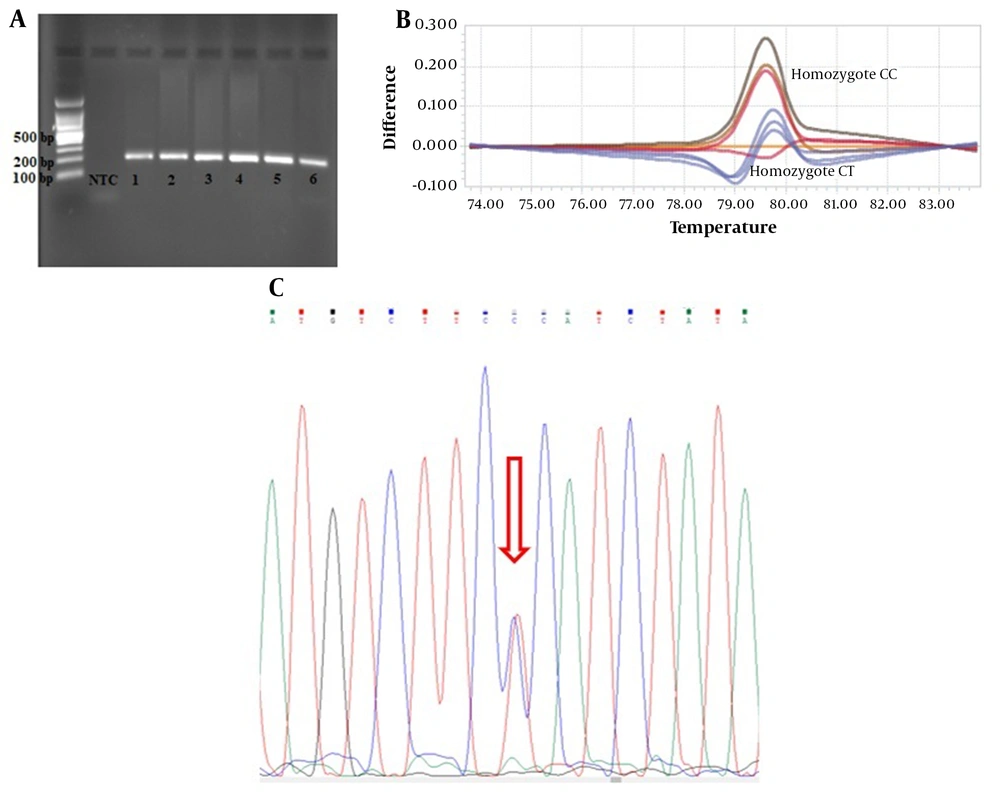
Two single nucleotide polymorphisms (SNPs) were chosen according to minor allele frequency based on the 1000 genome project from ARNTL and CLOCK genes. The rs6811520 with MAF = 0.2460 for the T allele is an intronic variant in the CLOCK gene (NM_004898.3:c.1450-143A>G) and also rs3789327 with MAF = 0.458 for the A allele is an intronic variant in the ARNTL gene (NM_001351824:c.460-1731A>G). Genomic DNA was extracted by PrimePrep Genomic DNA Isolation Kit (GeNet Bio, Cat. No. K-2000, Korea) from blood according to the manufacturer’s protocol. SNP genotyping was done using the Roche LightCycler® 96 System (Roche Life Science) by high resolution melting (HRM) method. The PCR reactions were performed in triplicate in 20 µL of the final volume containing 4 µL HRM master mix (Solis BioDyne), two μL primers, two μL of DNA, and 12 μL DEPC water (nuclease free). The PCR amplification protocol included an initial denaturation step at 95°C for five minutes, followed by 40 cycles of denaturation at 95°C for 30 seconds, annealing at 59°C for 30 seconds, extension at 72°C for 20 seconds, and finally, HRM included 95°C 60 s, 40°C 60 s, 65°C 1 s, and 97°C one second continuous. The allelic discrimination analysis was done with a different plot. The primer design was done by Gene Runner software (Table 1). Finally, for confirmation of HRM results, a random 20% of samples were amplified by PCR method and then sequenced using ABI 3130XL capillary sequencing platform (Applied Biosystems/Life Technologies, Carlsbad, CA, USA). The sequencing results were visualized as electropherograms and evaluated by chromas v. 2.6.6 Software.
| Polymorphism | Forward Primers | Revers Primers | Product Length (bp) |
|---|---|---|---|
| rs3789327 | 5'CACTACCAGCACCACAGAGC3' | 5'TAGGCAGACCGTACCAAGG3' | 106 |
| rs6811520 | 5'CACTGAAGTGATTCTCATTCCC3' | 5'GAGCTTGTTCAAGGTACTGTAGG3' | 199 |
Primers of the rs3789327 and rs6811520 Polymorphisms
3.4. Statistical Analysis
The chi-square test (χ2) was used between the MS patients and healthy control groups according to the significance of the difference with odds ratios (OR) and 95% confidence intervals (CI), to compare the genotype and allele frequencies. Also, an investigation of the Hardy-Weinberg equilibrium (HWE) in the groups was done by χ2 test. All employed analyses were done with SPSS, version 20.
4. Results
4.1. Epidemiologic and Demographic Results
In the present study, a total of 200 people participated in two groups, including MS patients and healthy control. The MS patient group included 63 females and 37 males with 32 ± 4.5 years of age. The female to male ratio was 1.7. In the control group, 58 women and 42 men healthy people with an identical ethnic background (mean age 35.5 ± 5.5 years) were selected.
4.2. HRM and DNA Sequencing Analysis
According to HRM analysis, the difference of the curves in the difference plot was observed in heterozygous and homozygous conditions at rs3789327 (Figure 1) and rs6811520 (Figure 2), and then HRM results were confirmed by Sanger sequencing (Figures 1 and 2).
4.3. Association Analysis of the rs3789327 and rs6811520 SNPs in MS Patients
The observed distribution of genotypes did not show a significant difference compared to the predicted Hardy-Weinberg equilibrium for patients and the control group (P > 0.05). The differences in genotype frequency and allele frequency of the CLOCK and ARNTL polymorphisms were assessed by χ2 test in the groups and displayed in Table 2. The frequency of genotype and allelic frequency of rs6811520 polymorphism was significantly different between the MS patients and healthy control groups (Table 2).
| Polymorphism | Genotype Frequency (%) | P-Value | Allele Frequency (%) | P-Value | OR | 95% Confidence Interval | |||
|---|---|---|---|---|---|---|---|---|---|
| rs6811520 | TT | TC | CC | T | C | ||||
| Case | 38 | 32 | 30 | 0.023 | 54 | 46 | 0.006 | 4.164 | 1.43 - 12.09 |
| Control | 44 | 17 | 39 | 52.5 | 47.5 | ||||
The Genotype and Allele Frequency of rs6811520
The genotype frequency in the MS patients for TT, TC, and CC alleles were 38%, 32%, and 30%, respectively, and that was 44%, 17%, and 39% in the healthy control group. The T allele frequency in the MS patients was higher than in the healthy control group (54% vs. 52.5%). Statistical analysis showed an increased risk of the TC genotype (P = 0.006, OR = 4.164, CI 95% = 1.43 - 12.09) in relation to the MS.
5. Discussion
Based on previous studies, we consider that genetic alterations in key genes that regulate circadian rhythms may play a role in the risk of developing MS. Indeed, genetic epidemiological data show considerably altered distribution of the CLOCK gene allele, genotype, and haplotype between world populations, which is interesting for health association studies (19, 20). A notable finding of our study is that the TC rs6811520 genotype in the CLOCK gene significantly increases the risk of MS. Ivana Skrlec et al.’s study revealed a relationship between myocardial infarction (MI) and polymorphisms of the CLOCK (rs6811520) and ARNTL (rs37389327) genes in a sample of 1057 participants. ARNTL rs3789327 was related to type 2 diabetes (T2DM) in MI patients, whereas the polymorphism of CLOCK rs6811520 was related to hypertension, T2DM, and systolic blood pressure (21). Lavtar et al.’s study assessed the ARNTL and CLOCK gene polymorphisms with multiple sclerosis. Totally, 900 Caucasian patients and 1,024 controls were compared. They found a significant difference in the genotype distributions of the ARNTL rs3789327 and CLOCK rs6811520 in the patients and the control group. The genotype CC of the ARNTL rs3789327 and the genotype CC of the CLOCK rs6811520 was linked with a higher risk for MS (19). Gyorik et al.’s study examined the impact of CLOCK variation on lifetime depression and current depressive symptoms. A significant association with SNPs rs6825994 and rs6850524 was identified with current depression in interaction with recent life events, whereas SNPs rs6828454 and rs711533 were identified in interaction with childhood adversity on current depressive symptoms (22). In Maciukiewicz et al.’s study, 42 polymorphisms of circadian clock genes, including PER3, ARNTL, CLOCK, and TIMELSSS, from 511 patients with bipolar affective disorder (BD) were genotyped. They detected an association between main depression and appetite disturbances with ARNTL variant rs11022778. In addition, an association was found between sleep disturbances with ARNTL rs11824092 and CLOCK rs11932595 (23). In Min et al.’s study, the correlation between rhythm gene polymorphisms and T2DM or BD was studied in the Han Chinese population. In total, 136 patients with BD, 166 patients with T2DM, and 130 healthy controls were selected for this study. The researchers studied polymorphisms in rhythm genes such as CLOCK and ARNTL. In the ARNTL gene, the allele frequencies of rs10832022-G and rs11022765-A were significantly higher in patients with T2DM than in patients with BD. The haplotype frequency of rs10832022-G, rs1022765-A, and rs11022762-T was considerably higher in patients with T2DM compared to patients with BD (24). Caroline E. Sutton et al.’s study found significant findings in myeloid cells that regulate Bmal1 clock gene expression and time-of-day, innate and adaptive immune responses mediated by autoimmune diseases. Their results show that mice with targeted deletion of Bmal1 significantly increased pro-inflammatory and T cell-polarizing cytokines, leading to increased inflammation and increased susceptibility to autoimmune diseases (25). It was suggested that disturbance in circadian rhythms might change cellular and molecular metabolic mechanisms that are arranged on a daily basis and so, contribute to nerve damage in the long term (26). Because cellular metabolic mechanisms are relevant to all health and disease conditions and are not specifically associated with nerve damage, circadian rhythms are key mechanisms that should be studied in chronic diseases.
5.1. Conclusions
Our data show that the frequency of genotypes and the frequency of rs6811520 polymorphism allele are significantly different between MS patients and the healthy control group. Our findings show no association between polymorphism rs3789327 and MS. The frequency of the T allele of rs6811520 polymorphism is higher in the MS group than in the control group. Statistical analysis shows an increased risk of MS associated with the TC genotype. Our results suggest that variation in the CLOCK gene is associated with MS. The potential limitation of our study was that it only studied the population of Iranian origin. Therefore, it is suggested that further studies should be performed on individuals with different genetic backgrounds with higher sample sizes.