1. Background
Stroke was the leading cause of over two-thirds of deaths related to neurosurgical pathologies in Vietnam and other middle-income nations. Neurosurgical pathologies account for one-fifth of both cause-attributable deaths and years of life lost due to disability (1). In the textbook, its anatomy is characterized by a symmetrical polygon of anterior and posterior circulation linked by connecting arteries, which provides several pathways for collateral blood flow to the brain (2). In patients without a history of cerebrovascular disease, incomplete circle of Willis (CoW) is related to the occurrence of circulation stroke, development of cerebral aneurysms, and their rupture in the future CoW (3-5). There is limited research in this area, especially in my country, Vietnam, although the CoW structure varied by ethnicity, and a survey of CoW revealed a fluctuating rate of variable completeness incidence (6, 7). Most previous studies in Vietnam were based on autopsies in the Cuc research (8), so the accuracy of the results and the medical infrastructure for imaging were limited, and the sample size was small.
2. Objectives
Consequently, we conducted this study to determine the diameters and classify the anatomical variations of the cerebral arteries in Vietnamese patients on MSCT 64 images more appropriately.
3. Methods
3.1. Participants
The research was conducted on patients who underwent 64- MSCT cerebral angiogram examination at Medical Imaging Department, Bach Mai hospital, between 7/2010 and 7/2011.
Inclusion criteria were as follows: Patients who were clinically diagnosed with cerebral hemorrhage, cerebral infarction, or suspected cerebrovascular disease and specialists confirmed they had not suffered any cerebral dissection, malformation of CoW, or its proximal branches after electroencephalogram, 64-MSCT, digital subtraction angiography or other diagnostic imaging angiograms. Patients aged 15 - 80 years, regardless of gender and location.
Exclusion criteria were as follows: Patients did not meet the above criteria. Patients had image noise, calcification of the vessel wall, and the level of stenosis of vessel diameter > 50%. Patients were treated with vascular intervention.
3.2. Study Design
A cross-sectional study was conducted.
3.3. Image Acquisition
By using a test bolus, it was possible to accurately determine when patients start receiving contrast agent in the cerebral arteries, record the appearance of the contrast agent, and predict the optimal delay time for the scanUpon completion of the test bolus. The patients underwent 64- MSCTs via a protocol consisting of two phases: The first phase evaluated the brain parenchyma, and the second studied the delayed background.
All information obtained would be transferred to the workstation for further evaluation: Delay background, reformat images by maximum intensity projection (MIP), measure the diameter of arteries, and identify variants of the CoW.
3.4. Image Analysis
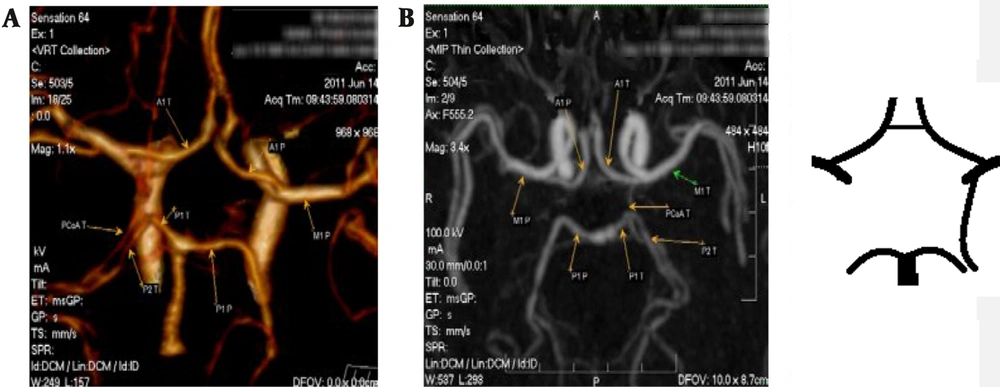
Following the acquisition of the dataset, images were reconstructed using a 10 mm MIP and volume rendering techniques (VRT). The cross-section is perpendicular to the circuit segment at three points: The starting point, the ending point, and the midpoint between the two mentioned positions. The documented arterial size is the average of these three locations. In our study, the mean diameter of the arteries was measured as the diameter of the artery lumen.
After measuring the diameter of the cerebral artery segments, we classified the variations according to the following types: Normal, hypoplasia, aplasia, and others. To classify the variations, we used the criteria of previous studies (9) and (10): Regular arterial segments ≥ 1 mm in diameter, absent segments as aplasia, and segments with < 1 mm diameter as hypoplasia.
The authors generally agreed with the standard that classified transformations after the CoW into adult configurations, fetal or embryonic configurations, and transitional configurations. Nevertheless, Al-Hussain et al. (9) accepts the above classification but adds specific comparative details. The classification of Al-Hussain et al. (9) is as follows: (1) adult configuration: The diameter of the pre communicating part P1 of posterior cerebral artery (PCA) (P1) segment of PCA is twice as large as posterior communicating artery (PCoA); (2) fetal configuration: the diameter of the PCoA is twice as large as the diameter of the P1 segment of the PCA; (3) transitional configuration: the diameter of the P1 segment of the PCA or the PCoA is not more than twice the diameters of each other.
3.5. Statistical Analysis
Data were analyzed by Stata 10.0 for Windows. Descriptive statistics were used to determine the ratio, the mean diameter of the vessels, and the frequency of variation in the study. Skewness/kurtosis test to check the normality of the dataset. Wilcoxon signed-rank test paired with pairs of non-normally distributed variables and parameterized t-test paired with normally distributed event pairs to compare mean diameters of circuit segments.
4. Results
4.1. Participants
All 102 Vietnamese patients met our inclusion criteria, consisting of 60 males and 42 females aged 15 - 80. The average age was 47. Of 102 patients, about half of the participants were middle-aged, between 37 and 58 years old.
4.2. Mean Diameter of Cerebral Artery Segments of the Circle of Willis
Table 1 shows the mean diameter of cerebral artery segments of the circle of willis.
| Variables | n | Mean Diameter | SD | Mean Diameter ± SD | Min | Max |
|---|---|---|---|---|---|---|
| A1 | ||||||
| L | 101 | 2.19 | 0.38 | 1.81 - 2.57 | 1.1 | 2.9 |
| R | 100 | 2.19 | 0.38 | 1.81 - 2.57 | 1.0 | 3.1 |
| P1 | ||||||
| L | 96 | 2.21 | 0.41 | 1.8 - 2.62 | 1.0 | 3.2 |
| R | 95 | 2.15 | 0.46 | 1.69 - 2.61 | 1.0 | 3.0 |
| PCoA | ||||||
| L | 53 | 1.62 | 0.50 | 1.12 - 2.12 | 1.0 | 3.0 |
| R | 48 | 1.67 | 0.48 | 1.19 - 2.15 | 1.0 | 2.5 |
| ACoA | 78 | 1.78 | 0.51 | 1.27 - 2.29 | 1.0 | 3.4 |
4.3. Anatomical Variations on the Image of 64- MSCT
Figures 1 and 2 show the anterior and posterior variations in the circle of Willis, respectively.
According to Al-Hussain’s classification, six forms of posterior variations of CoW are performed (Table 2).
| Posterior Variation in the Circle of Willis | Frequency (%) |
|---|---|
| Adult configuration | 55 (53.92) |
| Transitional configuration | 25 (24.51) |
| Transitional combined adult configuration | 9 (8.82) |
| Fetal combined adult configuration | 6 (5.88) |
| Fetal combined transitional configuration | 4 (3.92) |
| Fetal configuration | 3 (2.94) |
5. Discussion
5.1. Participants
A total of 102 photos were collected from 102 recruited subjects, 60 of whom were males, and 42 of whom were females. The average age of the participants was 47, ranging from 15 to 80. About half of the 102 patients were middle-aged, between the ages of 37 and 58. Based on the findings of (6), we focused on the middle-aged group since CoW incompleteness is common among middle-aged individuals.
5.2. Dimension of Arteries in the Circle of Wills
To determine the mean diameter more precisely, we calculated the average of 3 points: Starting, ending, and midpoint artery segment. There is not much difference between 3 points in the same artery segment; however, we suppose that indeterminate error would be repaired in comparison with these previous studies (8, 11, 12), which only documented 1 point in the artery segment (Table 3).
| Artery Segment | Our Result | Son in Vietnam (15) | Shatri et al. in Kosovo (13) | Karatas et al. in Turkey (14) |
|---|---|---|---|---|
| A1 | 2.09 ± 0.27 | |||
| L | 2.19 ± 0.38 | 2.26 ± 0.61 | ||
| R | 2.19 ± 0.38 | 2.15 ± 0.63 | ||
| ACoA | 1.78 ± 0.51 | 1.87 ± 0.91 | 1.5 ± 0.22 | 1.39 ± 0.83 |
| PCoA | 1.22 ± 0.2 | |||
| L | 1.62 ± 0.50 | 1.29 ± 0.63 | 1.27 ± 0.55 | |
| R | 1.67 ± 0.48 | 1.26 ± 0.66 | 1.30 ± 0.50 | |
| P1 | 1.99 ± 0.31 | |||
| L | 2.21 ± 0.41 | 2.37 ± 0.49 | 2.12 ± 0.52 | |
| R | 2.15 ± 0.46 | 2.37 ± 0.48 | 2.22 ± 0.67 |
a Values are expressed as mm.
We find that the mean diameter of the first segment of the anterior cerebral artery (A1) is nearly equal to (13) and (14).
Anterior communicating artery (ACoA): Son’s result (15) and we both recorded the mean diameter of ACoA larger than (13, 14). Vietnamese patients had a larger ACoA mean diameter than those in Kosovo and Turkey.
PCoA: The dimension of this cerebral artery segment is much greater than in the three mentioned studies. Compared to the three studies mentioned, this cerebral artery segment is much larger. The reason behind this can be explained by the fact that in the stage of research we conducted, there were too many technical limitations to accurately describe the size of a small vessel as PCoA - the thinnest segment of the polygon, compared to modern infrastructures, such as CT scan 256, MRA, CTA, which were used in these studies (13-15).
P1: Our findings are similar to the three studies.
As for the diametral difference between the two studies, this may be explained by the fact that we evaluated data based on Vietnamese patients suffering from cerebral diseases. In contrast, other studies collected data from the general population. It is also important to consider the size of the research.
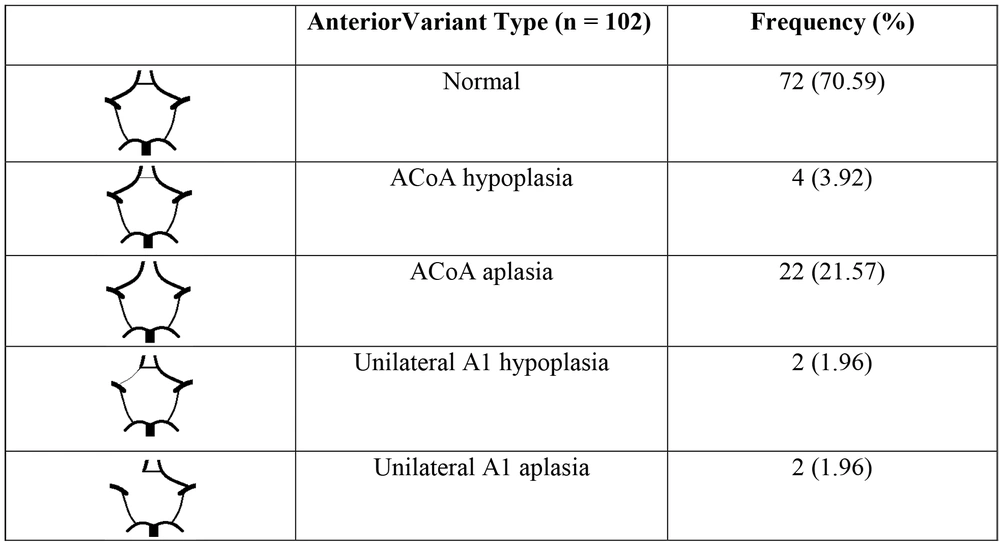
5.3. Anterior Variation in the Circle of Willis
The percentage of anterior variants in CoW in our research is 29.41%, greater than (10, 15, 16), less than (17, 18), and approximately similar to (19). We suppose the prevalence of variation is considerably different in each aforementioned study; it probably depends on many factors such as gender, method of investigation, race, environment, etc.
Anterior variants are most commonly found in ACoA. This form is also completely agreed upon in previous studies by (10, 14, 15, 17-19). As a result, patients who visit hospitals with stroke symptoms or for checkups should have their ACoAs checked initially in the anterior cerebral part. In contrast, Li et al.'s study (10) reported a variable frequency of 15.79%, significantly higher than our study and (15). This dissimilarity may be due to differences in sample size and racial factors. Thus, when approaching Chinese patients, we should be aware that complex variations of A1 should be considered more carefully than in Vietnamese patients.
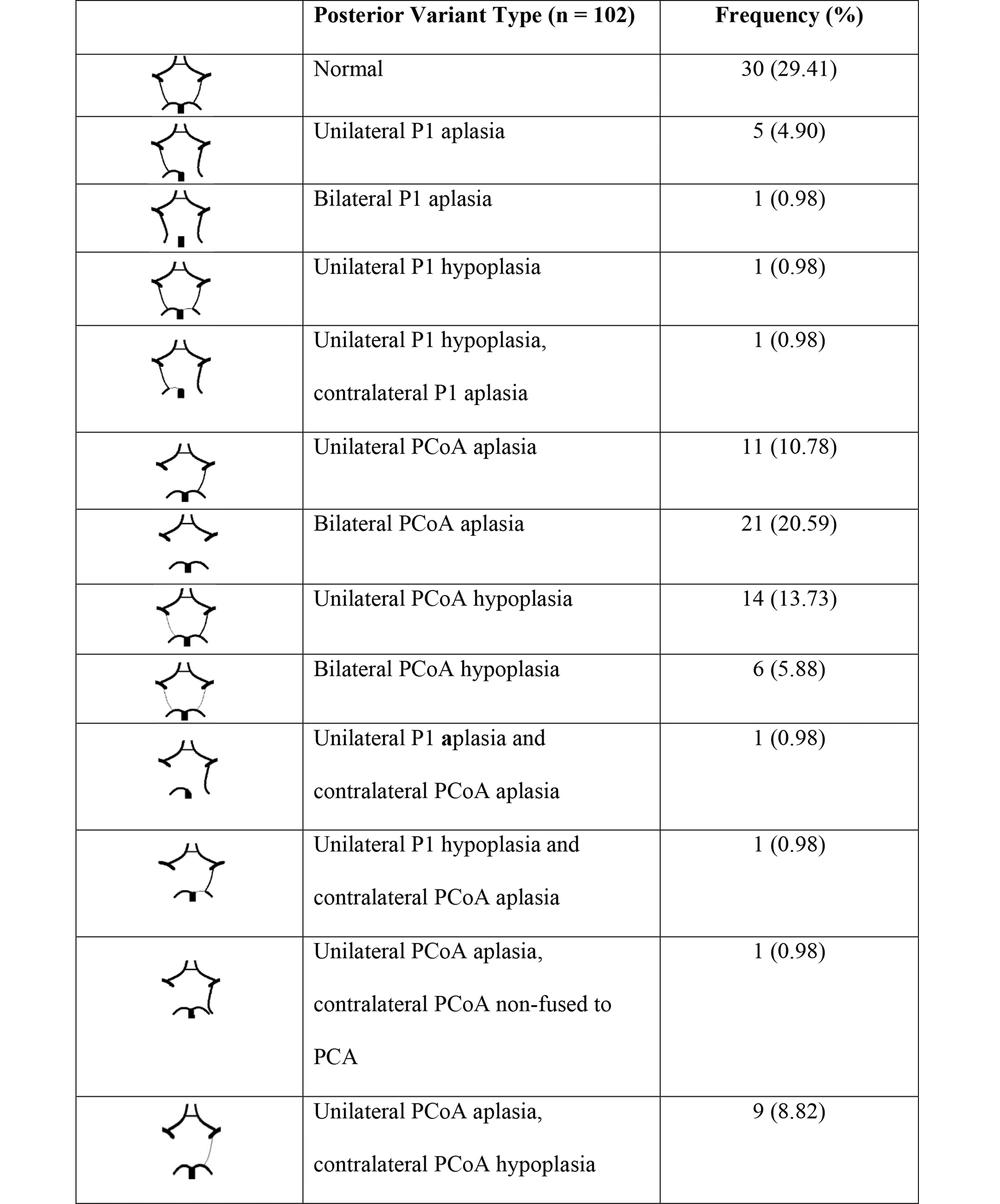
5.4. Posterior Variation in the Circle of Willis
The posterior variated model of polygon accounts for 70.59%. This proportion is similar to (16, 20, 21). Results indicate that the anatomical variation rate in the posterior part is higher and more varied than in the anterior part.
Our data indicate that PCoA (43.13%) is the most common variant, which is in agreement with most authors' conclusions (9, 10, 15, 19-21). While PCoA aplasia accounted for a greater share of total posterior variants in our study (10, 15, 21, 22), the opposite is true for (9, 23) since PCoA hypoplasia is predominant. Consequently, if the PCoA is not functioning, collateral circulation will be obstructed, leading to ischemic pathology (20).
In terms of variated configuration, we found that our study has a full range of forms as well as two additional types: Bilateral P1 aplasia, unilateral P1 aplasia, and contralateral PCoA aplasia. In addition, we documented all nine forms of posterior variation mentioned in (17). We have identified a new type of PCoA aplasia: unilateral PCoA aplasia and contralateral PCoA non-fused to PCA with just 0.98% of cases. A related variant was reported by Li et al. (10), but the result occurred only with unilateral PCOA non-fused to PCA without contralateral PCOA aplasia. A previous study by Cuc (8) published data in a similar form: Bilateral PCoA non-fused to PCA with 2.5%; they believe that there are two PCAs on each side. Nevertheless, from our perspective, there is one PCA on either side, and PCoA non-fused to PCA will appear as an event that changes the area of blood supply from PCA (Figure 3).
In prior research (24-29), the percentage of variant types varied widely, and our results are within this range. However, our documented proportion of the fetal group is lower than the previous range - from 11 to 40% (24-27), which may be explained by the frequent occurrence of hypoplastic PCoA and the significantly lower diameters of PCoA observed in our group. In fact that Vietnam has a lower fetal configuration; this variation may be an independent risk factor for PCoA aneurysms, predominantly in female patients (5).
Due to the low prevalence of fetal configuration in Vietnamese patients and the small mean diameter of PCoA, we believe that blood supply P2 is predominantly derived from the vertebral-basal artery system through the P1 segment of the PCA.
5.5. Limitations
Firstly, the images used in our study were evaluated by a single reader's observation and subjective evaluation. There is no specific agreement in the definition of anatomical variants of Willis polygon (some studies define hypoplasia as smaller than 0.8 mm, while others define it as smaller than 1 mm). Thirdly, the differences in imaging technologies and our data are directly compared to MRA, CTA.
5.6. Conclusions
Our findings identify the mean diameter of 7 main segment arteries of Willis polygon, four forms of anterior variation, and 12 forms of posterior variation, along with their frequency. According to the correlation between P1 and PCoA diameter, there are six variations in CoW and their frequency. As far as we are aware, this is the first paper that examines the configuration variants of the CoW through 64-MSCT in Vietnamese patients.
According to this study, this finding may be useful in planning and observing brain surgery, avoiding unexpected complications during intervention procedures, and estimating the prognosis of patients suffering from strokes or related cerebral vascular events.
Accordingly, we have found some similarities between the Vietnamese and Chinese populations, and further research should be conducted to determine whether racism and geography affect these characteristics.