1. Background
Coronaviruses are RNA viruses belonging to the family of Nidovirales and the order of Coronaviridae and are widely distributed in humans and other mammals (1).
Coronavirus can cause infection of various animal organs and cause more respiratory infections in humans. Although most human coronavirus infections are mild, epidemics of the two beta-coronaviruses, including severe acute respiratory syndrome coronavirus (SARS) and the Middle East respiratory syndrome (MERS), have caused infection of more than 10,000 cases in the past two decades, with 10 and 37% mortality rates for SARS and MERS that have been currently specified (2-8).
In December 2019, a series of pneumonia cases of unknown etiology appeared in Wuhan City, Hubei Province, China, with clinical manifestations similar to viral pneumonia. Consecutive in-depth analysis of lower respiratory tract samples revealed a new coronavirus, the novel coronavirus 2019 or coronavirus disease 2019 (COVID-19) (9-12). The outbreak was observed worldwide one month after the detection of the first case (13), and the route of transmission was expressed to be through human-to-human contact. The World Health Organization (WHO) declared COVID-19 an international public health emergency on February 1, 2020 (14). The disease was first manifested with severe respiratory characteristics in adults, and so far, there are few preliminary data on the incidence of this virus among children (15).
Clinical manifestations are the most accessible and reliable source of diagnosis (16). This disease's most common clinical manifestations include fever, cough, and dyspnea, with higher-grade fevers being more common in adults than children. Other clinical manifestations include muscle pain and fatigue, sputum production, sore throat, headache and diarrhea, hemoptysis, unwell, upper airway inflammation, runny nose, nausea, and vomiting.
Among common laboratory findings of this disease, one can mention the reduction of lymphocytes and albumin and an increase in levels of bilirubin, creatinine (CR), lactic dehydrogenase (LDH), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), creatinine kinase (CK), aspartate aminotransferase (AST), alanine aminotransferase (ALT) as well as leukocytosis, leukopenia, anemia, and thrombocytopenia (11, 17-19). Clinical manifestations are one way to diagnose this disease (14).
Many patients with COVID-19 progress rapidly from the early stages of the disease to the severe stages, so there is an urgent need to help frontline clinicians effectively triage patients during the COVID-19 pandemic (15).
Our information on the relationship between demographic characteristics, clinical manifestations, and laboratory findings of patients with COVID-19 and its relationship with severe outcomes can give us an insight into the underlying pathological mechanisms of the disease. In this case, decision-makers can manage patients more efficiently and prevent patient mortality when the disease is more prevalent, and they face a shortage of ICU beds (20).
2. Objectives
Since most studies have identified the clinical manifestations and laboratory findings of COVID-19 and a small number of studies have examined the relationship between these cases and severe outcomes of the disease, the researchers decided to conduct the present study to identify clinical manifestations and laboratory findings of COVID-19 patients and their relationship with severe outcomes. Thus, it is also hoped that the findings of the present study will make the international community and the country's authorities more familiar with the features, clinical symptoms, and laboratory results of this emerging disease and its relationship with severe outcomes (ICU admission and death) and help them to identify the virus and the resulting complications to improve control, prevention, diagnosis, treatment and decrease mortality of the disease.
3. Methods
This cross-sectional study with descriptive and analytical purposes was conducted as a single-center study, and the study population included all patients with confirmed COVID-19 admitted to the medical center affiliated with the Dezful University of Medical Sciences. The prevalence of COVID-19 was high in this city at the time of the study for several months. Clinical manifestations and laboratory test results of these patients were assessed.
The study was performed after obtaining permission from the Vice Chancellor for Education and Research, the Ethics Committee of the Dezful University of Medical Sciences, and the officials of the Ganjavian Hospital. In this study, medical cases were used. After referring to the medical cases of patients whose hospitalization date was from April to June 2020, 470 documents were selected from 1,247 documents by simple random sampling, and their information was extracted. Inclusion criteria included the patients with confirmed COVID-19 based on the doctor's diagnosis and positive result of the polymerase chain reaction (PCR) test and all the inpatients. Exclusion criteria included patients' medical records with not three follow-up laboratory tests in their records in the first to fifth days of hospitalization. Three follow-up sessions were held to investigate changes in laboratory tests. The data included clinical manifestations and the results of the first three patients’ laboratory test appointments (during the first five days of hospitalization). Patients' information was collected by observing the principles of confidentiality and anonymity.
Data included clinical manifestations and three laboratory test findings. anonymity and confidentiality of participants' information were considered.
For researchers, the criteria were three laboratory tests, including patients' information collected by observing the principles of confidentiality and anonymity. On the other hand, researcher criteria in this research were patients who had three laboratory tests with the variables under study investigation in the first five days of hospitalization, which could be completed in the first three or some patients in 1, 3 and 5 days of hospitalization and therefore, on the fifth day, three tests were completed.
Clinical manifestations refer to any signs and symptoms mentioned in the patient's record from admission day to discharge or death. Because the range of changes in these features was very diverse, it was attempted to consider all clinical manifestations, even those that might not have been observed in previous research. In this study, the means of severe outcome is considered ICU admission and death.
Descriptive statistics analyzed the data. Chi-square, Fisher's exact, Friedman, Mann-Whitney U, and Wilcoxon signed-rank tests were used using SPSS software version 22, and P-values smaller than 0.05 were considered statistically significant.
4. Results
A total of 470 patients҆ records were investigated in the present study. The average age of the patients was 56.5 ± 18.2 years old. The minimum and maximum age range was equal to 16 and 99 years old, respectively. The mean age was 66.7 years old among patients with severe outcomes (ICU admission and death) and 53.7 years old among those without severe outcomes. The Mann–Whitney U test results showed that age was significantly higher in the patients with severe outcomes than in the other group (P = 0.001).
Among the subjects, 53.6% (n = 252) of the subjects were male, and 46.4% (n = 218) were female, there was no significant relationship between sex and severe outcome (P = 0.784).
Study results about underlying disease showed, respectively, no history of underlying disease (56.4%, n = 265), heart disease (27%, n = 127), diabetes (13.4%, n = 63), neurological disease (2.5%, n = 12), and respiratory disease (0.63%, n = 3) among patients. The results showed that people with the underlying disease were 7.7 times more likely to experience severe outcomes, such as death or ICU admission (CI = 4.5 - 13.1) than those without underlying disease.
Sixty-four (13.6%) of the patients had a history of previous hospitalization, and 86.4% (n = 406) of them had no history of previous hospitalization (from birth to now for any reason). There was no relationship between the history of any previous hospitalization and the severe outcome of the disease (P = 0.993).
Most of the patients (86.4%) lived in cities (n = 406), and 13.6% (n = 64) of them lived in villages, and there was no relationship between the place of residence (urban/rural) and severe outcome of the disease (P = 0.510).
Among the patients, 94% (n = 442) were married, and 6% (n = 28) were single. There was a statistically significant relationship between marital status (married/single) and the severe outcome of the disease, and all the married patients experienced a severe outcome (P = 0.004).
The mean ± SD of hospital stay was equal to 8 ± 2 days. Results of the Mann–Whitney U test also showed no statistically significant difference between the two groups of patients with severe outcomes (ICU admission or deceased patients) and other patients in terms of mean days of hospitalization (P = 0.743).
Among the clinical manifestations, dry cough (95%, n = 448), fever (93%, n = 437), and dyspnea (67%, n = 315) were the most common clinical manifestations in the patients. The frequency of other clinical manifestations was as follows: Chills (47%, n = 222), chest pain (44%, n = 206), fatigue (26%, n = 123), headache (24%, n = 111), vertigo (24%, n = 114), sputum production (24%, n = 114), diarrhea (18%, n = 89), rhinitis (14.6%, n = 69), nausea (10%, n = 48), vomiting (10%, n = 49), myalgia (10%), n = 47), sore throat (6%, n = 29), anosmia (5%, n = 21), ageusia (n = 22), and runny nose (5%, n = 24).
There was a statistically significant relationship between fever (P = 0.012), chills (P = 0.031), nausea (P = 0.044), and severe outcomes (ICU admission or death).
Regarding the need for oxygen, the patients included people who needed oxygen therapy with a mask (74.5 %, n = 350), ventilator (20.6 %, n = 97), nasal cannula oxygen therapy (3.4 %, n = 16), and those not needing oxygen therapy (1.5 %, n = 7). Results also showed that people who needed oxygen therapy during the treatment process (nasal cannula, mask, and ventilator) significantly experienced more severe outcomes (ICU or death) than other patients (P = 0.001).
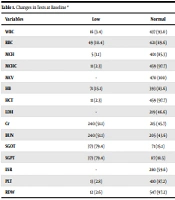
The results of baseline tests (first session) are shown in Table 1. There was a statistically significant relationship between test results of hemoglobin (HB) (P = 0.006), red blood cells҆ distribution width (RDW) (P = 0.011), platelet count (PLT) (P = 0.00), blood urea nitrogen (BUN) (P = 0.048), creatinine (CR) (P = 0.026), and lactic dehydrogenase (LDH) (P = 0.044) and severe outcomes (death and ICU admission).
| Variables | Low | Normal | High | Total |
|---|---|---|---|---|
| WBC | 16 (3.4) | 437 (93.0) | 17 (3.6) | 470 (100) |
| RBC | 49 (10.4) | 421 (89.6) | - | 470 (100) |
| MCH | 5 (1.1) | 401 (85.3) | 64 (13.6) | 470 (100) |
| MCHC | 11 (2.3) | 459 (97.7) | - | 470 (100) |
| MCV | - | 470 (100) | - | 470 (100) |
| HB | 71 (15.1) | 393 (83.6) | 6 (1.3) | 470 (100) |
| HCT | 11 (2.3) | 459 (97.7) | - | 470 (100) |
| LDH | - | 219 (46.6) | 251 (53.4) | 470 (100) |
| Cr | 240 (51.1) | 215 (45.7) | 15 (3.2) | 470 (100) |
| BUN | 240 (51.1) | 205 (43.6) | 25 (5.3) | 470 (100) |
| SGOT | 373 (79.4) | 71 (15.1) | 26 (5.5) | 470 (100) |
| SGPT | 373 (79.4) | 87 (18.5) | 10 (2.1) | 470 (100) |
| ESR | - | 280 (59.6) | 190 (40.4) | 470 (100) |
| PLT | 13 (2.8) | 410 (87.2) | 47 (10.0) | 470 (100) |
| RDW | 12 (2.6) | 547 (97.2) | 1 (0.2) | 470 (100) |
Changes in Tests at Baseline a
Mean changes in blood tests in the first three sessions, including baseline, second, and third sessions, were evaluated using Friedman and Wilcoxon Signed Rank tests. The results showed significant changes in the test results of white blood cells (WBC), BUN, CR, mean corpuscular volume (MCV), mean corpuscular hemoglobin concentration (MCHC), HB, PLT, CRP, ESR, LDH, and serum glutamic pyruvic transaminase (SGPT) between the first and third sessions and these changes were non-significant in the test results of red blood cells (RBC), mean corpuscular hemoglobin (MCH), hematocrit (HCT), RDW, and serum glutamic oxaloacetic transaminase (SGOT) (Table 2). Table 3 shows different medications prescribed for these patients for the duration of hospitalization but not simultaneously (Table 3).
| Variables | Time | P-Value a | Comparison | P-Value b | ||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ||||
| WBC | 7.66 (1.50) | 7.47 (1.61) | 7.44 (1.63) | 0.001 | 1 - 2 | 0.001 |
| 1 - 3 | ||||||
| 2 - 3 | ||||||
| MCV | 88.31 (4.98) | 87.48 (9.47) | 88.02 (6.39) | 0.001 | 1 - 2 | 0.847 |
| 1 - 3 | 0.001 | |||||
| 2 - 3 | ||||||
| MCHC | 33.42 (1.16) | 33.26 (2.47) | 33.27 (4.47) | 0.004 | 1 - 2 | 0.018 |
| 1 - 3 | 0.4 | |||||
| 2 - 3 | 0.016 | |||||
| HB | 13.47 (1.10) | 12.71 (1.74) | 11.87 (1.91) | 0.001 | 1 - 2 | 0.001 |
| 1 - 3 | ||||||
| 2 - 3 | ||||||
| PLT | 320.1 (100.28) | 292.94 (111) | 281.95 (101.25) | 0.001 | 1 - 2 | 0.001 |
| 1 - 3 | ||||||
| 2 - 3 | ||||||
| CRP | 2.27 (1.3) | 1.97 (0.84) | 2.22 (0.83) | 0.001 | 1 - 2 | 0.24 |
| 1 - 3 | 0.076 | |||||
| 2 - 3 | 0.001 | |||||
| ESR | 20.3 (10.1) | 35.91 (15.07) | 49.45 (25.26) | 0.001 | 1 - 2 | 0.001 |
| 1 - 3 | ||||||
| 2 - 3 | ||||||
| BUN | 7.62 (8.24) | 5.31 (7.84) | 6.62 (8.24) | 0.001 | 1 - 2 | 0.001 |
| 1 - 3 | 0.038 | |||||
| 2 - 3 | 0.002 | |||||
| CR | 0.48 (0.49) | 0.34 (0.47) | 0.48 (1.03) | 0.001 | 1 - 2 | 0.004 |
| 1 - 3 | 0.45 | |||||
| 2 - 3 | 0.22 | |||||
| LDH | 494.76 (125.39) | 606.96 (171.24) | 720.79 (356.88) | 0.001 | 1 - 2 | 0.001 |
| 1 - 3 | ||||||
| 2 - 3 | ||||||
| RBC | 4.43 (0.32) | 4.41 (0.47) | 4.41 (0.42) | 0.44 | ||
| MCH | 29.79 (2.48) | 29.57 (3.39) | 29.65 (3.12) | 0.81 | ||
| HCT | 38.69 (2.65) | 41.81 (36.09) | 38.50 (3.66) | 0.55 | ||
| SGOT | 6.39 (14.35) | 6.84 (15.14) | 9.25 (17.97) | 0.146 | ||
| RDW | 12.57 (1.01) | 12.50 (1.41) | 12.52 (1.30) | 0.714 | ||
Changes in the Blood Tests Between Baseline, Second, and Third Sessions
| Medication | No. (%) |
|---|---|
| Chloroquine | 470 (100) |
| Hydroxy chloroquine | 470 (100) |
| Oseltamivir | 470 (100) |
| IVIG | 470 (100) |
| Lopinavir/ritonavir | 229 (48.72) |
| Azithromycin | 469 (99.78) |
| Naproxen | 469 (99.78) |
| Acetaminophen | 459 (97.65) |
| Anti-coagulants | 72 (15.32) |
| Electrolytes | 447 (95.1) |
| Zinc | 306 (65.1) |
Medications Prescribed for Patients in Duration of Hospitalization
5. Discussion
In the present study, clinical manifestations and laboratory findings of patients with confirmed COVID-19 were investigated in a city in Iran (Dezful). In this regard, many studies have been conducted in different parts of the world. Results of the present study revealed a statistically significant relationship between age and severe outcome, i.e., ICU admission or death (P = 0.001). Many studies performed in different parts of the world have reported similar results regarding the high prevalence of COVID-19 in elderly patients (12-14, 21-23) and the relationship between age and death related covid-19 (24).
Unlike younger people, elderly people are at a higher risk of infection with COVID-19 due to their weaker immune systems. Also, they often have chronic underlying diseases and more severe infections; consequently, the mortality rate is higher among them (25).
This increased risk is due to several age-related biological, clinical reasons, and environmental effects, all of which cause changes in the immune system, including changes in cytokine response to immune activators, changes from (cell-mediated) type 1 cytokine response to (humoral-mediated) type 2 cytokine response with the increase in age as well as expression of chronic pro-inflammatory cytokines, which is partly due to the increased presence of old cells, as well as disruption of phagocytosis by macrophages and dendritic cells, and a change in toll-like receptor (TLR) (26).
On the other hand, findings have indicated that the elderly are unable to take measures, such as isolating themselves and are weak at adhering to preventive measures, especially using face masks in outdoor places. These behaviors become important when social distance is not observed (27).
Despite all these studies, some studies have reported conflicting results. Contrary to the results of our study, the results of another study have demonstrated that the COVID-19 epidemic affected more young people in the United States during June - August than in January - May 2020 (28).
There was also a similar age change in Europe, where the average age of COVID-19 cases decreased from 54 years of age during January - May to 39 years of age during June - July, when people aged between 20 - 29 years old accounted for the highest proportion of cases (19.5%) (29, 30).
Results of the present study showed no statistically significant relationship between sex and severe outcomes in the subjects under study consisting of 53.6% of males and 46.4% of females (P = 0.784). However, many studies have reported that being male is associated with experiencing severe disease outcomes (13, 18, 23, 31, 32).
Although some studies showed women are more immune to viral infections (33), but the results of our study did not show a statistically significant difference between gender and disease outcomes.
Our study's first underlying disease was cardiovascular disease (27%). Numerous studies have investigated the effects of heart disease. The pre-existing cardiovascular disease seems to be associated with severe outcomes and an increased risk of death among patients with COVID-19. Findings from our study and several others can confirm that COVID-19 is more likely to cause myocardial damage through inflammation, resulting in cardiac dysfunction and life-threatening arrhythmias (28, 34), acute coronary syndrome, and venous thromboembolism. Potential drug-disease interactions influencing patients with COVID-19 and associated cardiovascular diseases have also become a serious concern (28).
In the present study, diabetes (13.4%) was the second underlying disease associated with severe outcomes. In addition to circulatory diseases, endocrine diseases, such as diabetes, were also commonly seen among patients with COVID-19. Patients with comorbidities suffered from a more severe COVID-19 outcome than those with no comorbidities. In this study, kidney diseases, malignancies, and pregnancy were also mentioned, which can be due to the large sample size (18).
However, the association between different underlying diseases and prognosis was less consistent in some studies on the coronavirus family. For example, studies evaluated the relationship between heart disease and poor clinical outcomes in influenza, SARS-CoV, or MERS-CoV have reported no definite result, or there was no disease, except for diabetes, predicting poor clinical outcomes in patients with MERS-CoV infection (11, 18, 25, 35-38). Our results showed a positive and significant association between cerebrovascular disease and the severe outcome of COVID-19 with less frequency (2.5%).
The presence of underlying diseases, such as cerebrovascular damage, may increase the severity of infectious diseases, which is true for COVID-19 due to its pathomechanisms (39, 40).
Herein, a positive and significant association was also found between respiratory disease and severe outcomes of COVID-19 with less frequency (0.63%). The effect of comorbidities, including respiratory disease, has also been reported in a similar study (P = 0.0007). According to the results of this study, underlying diseases, including respiratory disease, were compared in surviving patients and those who died from COVID-19. The results showed a significant relationship between both groups, which was significantly higher in deceased patients (23).
In a study on symptoms in patients, a similar number of symptoms were reported in inpatients and outpatients, and the most common symptoms were fever (68%), cough (69%), dyspnea (72%), chills (60%), fatigue (65%), and body ache (56%). In our study, the most common symptoms included dry cough (95%), fever (93%), and dyspnea (67%), respectively. According to the report in this study, the inpatients described dyspnea and frequent respiratory problems (P < 0.001). However, our findings revealed a significant relationship between fever, chills, nausea, and severe outcomes (38). But perhaps what is more important in presenting these results is the duration of the fever, which was not considered in our study. In a systematic review and meta-analysis of clinical manifestations, risks, and outcomes of COVID-19, abdominal pain was introduced as a rare symptom significantly associated with severe COVID-19; thus, those with abdominal pain were controlled as soon as possible. However, abdominal pain was not reported by patients in the present study. In that systematic review study, gastrointestinal involvement (nausea, vomiting, and abdominal pain) and respiratory symptoms (dyspnea and chest pain) were associated with severe COVID-19 outcomes. In our study, nausea was also one of the symptoms associated with severe outcomes of the disease; however, among other features in our study, instead of respiratory symptoms, there was a significant association between fever, chills, and nausea with severe outcomes of the disease.
In the present study, in the baseline laboratory test results, increments and decrements were observed in WBC, MCH, HB, RDW, PLT, BUN, CR, SGOT, and SGPT in different patients. Also, there was a decrement trend in experimental values of HCT, RBC, and MCHC and an increment trend in ESR and LDH. MCV was normal in all patients. There was a statistically significant relationship between HB, RDW, PLT, BUN, CR, and LDH and severe outcomes (death and ICU admission). Also, changes in blood test results in the first three sessions were evaluated, and the results showed significant changes in the laboratory findings of WBC, MCV, MCHC, HB, PLT, CRP, ESR, BUN, CR, LDH, and SGPT between the first to third sessions. Other studies have also investigated the laboratory findings of COVID-19. For example, results of another study showed higher levels of CRP among the elderly in patients with chronic underlying diseases, especially cardiovascular diseases, and levels of WBC, neutrophils, and lymphocytes were lower in these patients than in other patients (22).
In other studies, high levels of LDH were reported to be significantly associated with severe COVID-19 outcomes. Survival analysis also showed that high leukocytosis and LDH levels were associated with a higher mortality rate in patients with severe COVID-19 outcomes (41). High LDH levels in severe COVID-19 cases may indicate an association between LDH and lung and tissue damage (42, 43). Increased LDH indicates cell death and injury and is associated with a poor host immune response, resulting in higher susceptibility to severe viral infections (42, 44, 45).
Other studies, like the present study, have also shown that some common laboratory biomarkers, which exceeded the reference range, were associated with more severe disease. Reviewing the results of these studies demonstrated significant differences between patients with mild and very severe COVID-19 outcomes in terms of leukocyte count, lymphocyte count, total protein, total bilirubin, LDH, BUN, D-dimer, CRP, and ferritin (43).
However, it should be noted that these biomarkers are not all specific to SARS-CoV-2. However, the increased level of inflammatory biomarkers, such as CRP and ESR, indicates that the pathogenesis of the very severe SARS-CoV-2 infection is associated with the impaired inflammatory response (18). However, some other studies have reported an increase in WBC count and a decrease in lymphocytes and platelet count in patients with severe and deadly diseases compared to those with non-severe diseases. Studies have also reported increased ALT, AST, BUN, CR, and coagulation test levels in patients with severe and deadly COVID-19 (32, 46).
In our study, the highest respiratory support was supplied by face masks (74.5%), followed by nasal cannulas (3.4%). Another study investigated the clinical manifestations of adult inpatients with COVID-19 in a healthcare system in California (USA). Results showed that most patients received oxygen therapy through a cannula or face mask (2).
In the present study, the mean ± SD of hospital stay was equal to 8 ± 2 days. The Mann-Whitney U test results also showed no significant difference between the two groups of patients with severe outcomes (ICU-hospitalized or dead patients) with other patients regarding the average length of hospital stay. However, results of a study conducted in China reported a mean hospital stay of 24 days for all the patients (range = 5 - 50 days), 36 days for critically ill patients, and 18 days for non-critically ill patients (47). This discrepancy between the results of the studies may be attributed to the patients҆ consciousness, early referrals, and conditions governing the society regarding COVID-19 pandemic policymaking and management in China (36). There is a relationship between the spread of the virus and different variables in different geographical areas. Therefore, various variables can be analyzed in the future challenges; for example, the date when the first COVID-19 case was identified in the country and studying humidity, temperature, and other meteorological variables, as well as other types of variables in different regions, such as cultural behavior, religious behavior, healthcare-related habits, eating habits, etc., which may influence the incidence or severity of the disease (48).
5.1. Conclusions
Covid-19 has rapidly spread and often progresses among people. In the world, we have encountered limited ICU units and a limitation of hospital beds; thus, using some factors, such as clinical manifestation and laboratory tests, gives an early warning for rapid interventions and decreases the number of deaths and ICU admissions of these patients. So, considering the main symptoms of COVID-19, such as fever, cough, fatigue, and dyspnea, can have a key role in the early detection of this disease. However, the data from this study should be interpreted with caution.