1. Background
Schistosomiasis is a chronic parasitic and often weakening disease, commonly known as Bilharziosis, which is caused by trematodes of the genus Schistosoma (1, 2).
The disease appears in two clinical forms, intestinal and genitourinary. Intestinal Schistosomiasis is caused by S. mansoni, S. japonicum, S. macungi, and S. intercalatum species, and genitourinary Schistosomiasis is caused by S. haematobium, respectively (3). Schistosoma parasite is transmitted to the human as a definitive host through an intermediate host of snails (4).
The distribution of the disease mainly depends on the presence of Biomphalaria spp. and Bulinus spp., which are intermediate host snails for S. haematobium and S. mansoni (5). The distribution of the disease mainly depends on the presence of Bulinus spp. and Biomphalaria spp., which are intermediate host snails for S. haematobium and S. mansoni, respectively (5).
Besides the distribution of intermediate hosts snails, human hygiene and contact with contaminated water also play an important role in the transmission of Schistosoma. The intensity of infection is influenced by the frequency of water contact and persistence in contaminated water (6, 7). Factors such as age, sex, occupation, education level of the head of the family, religion, and place of residence can affect the person's contact with contaminated water (8-11). Schistosomiasis is more common among schoolchildren (12). The distribution of the disease in an endemic area has been attributed to greater contact with contaminated water among school children between 10 and 15 years old (13, 14).
Since 2014, 258 million people have been diagnosed with Schistosomiasis (15). This disease has infected 207 million people in 76 countries. It is endemic in 52 countries, mainly in developing countries in Asia, Africa, South and Central America, and the Middle East, where almost 90% of people need treatment (16, 17). Eight hundred million people are also at risk of developing this disease, with at least 280 thousand people endangered of dying each year (16). Schistosomiasis also causes disability-adjusted life years (DALYs) among more than 70 million people (18, 19).
2. Objectives
The aim of this study was to determine the prevalence of Schistosomiasis in the north of Khuzestan province, which was one of the most important foci areas of Iran for this disease. In light of the fact that infected countries with Schistosomiasis must prepare a document of the disease, this study can be regarded as a document to declare the elimination of the disease to the World Health Organization (WHO).
3. Methods
3.1. Area of the Study
The areas of Dezful, Shush, and Gotvand are located in the north of Khuzestan province (23°056 ~ 30°38′E, 35°37 ~ 36°28′N), southwest of Iran. This region has a semi-arid climate with an average temperature of 22.8 degrees Celsius (the maximum temperature in the hottest month is 53 degrees Celsius, and the minimum temperature in the coldest month is 4 degrees Celsius), the average annual rainfall is 447.9 mm, and the relative humidity is 44.5%. According to the latest national census, 2015, its population is 786,415 people, with an area of 9,586 square kilometers; three main permanent rivers pass through these areas, including Dez, Shavor, and Karkheh.
3.2. Plan Study and Population
This cross-sectional study was conducted in 2017 in three areas of Khuzestan province, including Dezful, Shush, and Gotvand. Year six of primary school and the first year one of the secondary school students (under 15 years old) from these regions were present in the study.
3.3. Sampling and Sample Size
The samples were selected from 16 regions identified as being at high risk of the disease based on 40-year-old records of the disease using a multi-stage random cluster sampling method.
There were 24 clusters (schools) in these 16 regions, and all these schools participated in the plan, including 5 clusters in Dezful, 16 clusters in Shush, and 3 clusters in the Gotvand areas. Then, from these schools, in Year six of primary school and the first year, one of the secondary school students (under 15 years old) was selected by census method, and in the next stage, the students were randomly selected. 483 male and female students were selected from urban and rural areas endemic to the disease.
3.4. Inclusion Criteria
The selected students were from endemic areas and had been living there from birth, whose parents have expressed their consent for their child's participation in the study.
3.5. Exclusion Criteria
The criteria were: Students older than 15 years, not living since birth in endemic areas of the disease, and lack of consent to participate in the study.
3.6. Data Collection Tools
- Data were collected through a checklist. The checklist had 4 sections, including: (1) demographic information (age, gender, number of family members, and accommodation in an infected area); (2) disease symptoms (burning and pain during urination, histories of hematuria in the referred case disease, and member families); (3) contact behavior with water (swimming in the river or irrigation channel, washing clothes in the river or irrigation channel, and farming); and (4) history of travel to Iraq.
- Blood sampling (5 mL) and urine sampling (150 mL) from the students were carried out.
Urine sampling was based on the "National Guidelines for Care Schistosomiasis in Iran" (20), and blood sampling was according to the guidelines of laboratory diagnostic kits.
The blood samples were sent to the medical laboratory of Imam Khomeini (RA) hospital in Dezful city, and after separating the serum, serum concentrations of IgG of samples were measured by the LTD kit (Human Schistosoma Elisa Kit; SHANGHAICRYSTAL DAY BIOTECH CO, LTD, Cat. No: ED4390Hu) which was made by a German company according to the manufacturer's instructions.
A Leitz light microscope was used to detect the parasite ova of S. haematobium based on urine samples sent to the medical laboratory of Shush and Dezful hospital. In order to observe parasite eggs in urine, the direct sedimentation method was used by the laboratory technician.
At least 150 cc of urine was collected from each person from 11:00 (AM) to 2:00 (PM). The samples were transferred to the laboratory on the same day (to avoid changing the parasite eggs). They were poured into conical glass containers and kept in the refrigerator for 24 hours, and after sedimentation, the surface of the urine was discarded, and the last third was examined (20).
3.6.1. Human Schistosoma ELISA Kit
Schistosoma (Schistosoma) was assayed in a human serum sample and blood plasma using this kit. The kit uses a double-antigen sandwich enzyme-linked immunosorbent assay.
Summary procedures: (1) preparing reagents, samples, and standard; (2) adding prepared sample and standard, incubating 30 minutes at 37°C; (3) plate washed five times, adding HRP-conjugate reagent incubated 30 minutes at 37°C; (4) plate washed five times, adding Chromogen solution A and B incubated 10 minutes at 37°C; (5) adding stop solution; and (6) measuring within 15 min.
3.6.1.1. Result Determinant
- Test validity: The average of the positive control well > 1.00; the average of the negative control well < 0.10.
- Calculate critical (CUT OFF): Critical = the average of Negative control well +0.15.
- Negative control: Sample OD < calculate critical (CUT OFF) is Schistosoma negative control.
- Positive control: Ample OD > calculate critical (CUT OFF) is Schistosoma positive control.
3.7. Statistical Analysis
In order to investigate the demographic variables, descriptive statistical methods were used, and to investigate the significant relationship between the variables, the chi-square test was used with SPSS version 16 software.
3.8. Methods of Surveying
This research study was a cross-sectional type. The studied cases included 483 male and female students in the age group of 10 to 15 years. The place of residence of all these students was in the disease-endemic areas of Dezful, Shush, and Gotvand cities located in the north of Khuzestan province (23°056 ~ 30°38′E, 35°37 ~ 36°28′N) in the southwest of Iran.
This region has a semi-arid climate and an average temperature of 22 (Karkheh) eight degrees celsius (the maximum temperature in the hottest month is 53 degrees celsius, and the minimum temperature in the coldest month is 4 degrees celsius), the mean annual rainfall is 447.9 mm, and the relative humidity is 44.5%. According to the latest national census, its population was 786,415 in 2015, with an area of 9,586 square kilometers, and three permanent rivers pass through this area, including Dez, Shavor, and Karkheh.
Urine samples of the studied student cases were taken from 11:00 (AM) to 2:00 (PM) to detect S. haematobium ova through observation of the 24-hour sediment under the light microscope and measuring serum samples to check for antibodies against the studied S. haematobium infection. This study was carried out in 2017 (20).
Demographic characteristics, information on risk factors, and the history of the presence of disease symptoms in the past and present, as well as the history of traveling to Iraq, a neighboring country and endemic for the disease, were collected through face-to-face questions from students based on a questionnaire. Finally, the data analysis, including the descriptive and analytic statistical methods, was carried out using SPSS version 16 software.
4. Results
4.1. Prevalence of Schistosoma haematobium
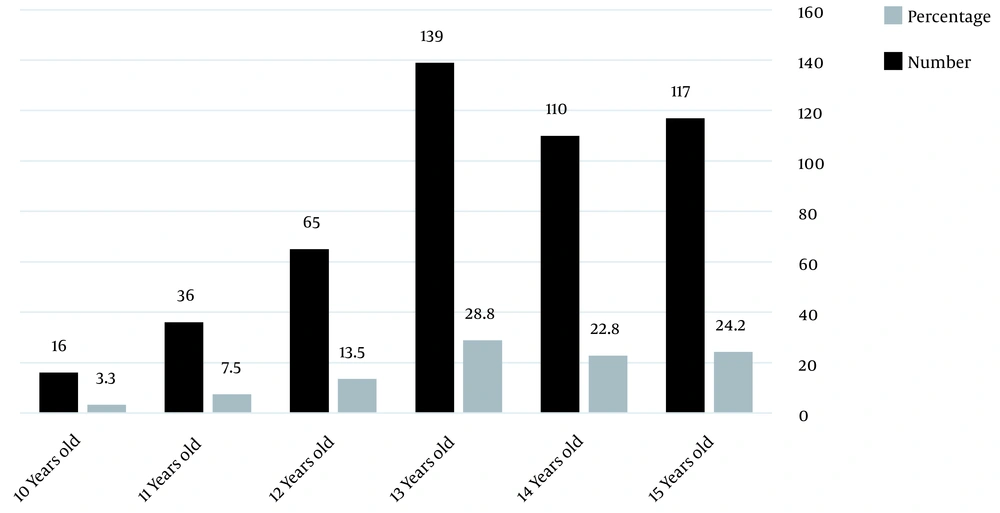
A total of 483 students aged 10 - 15 years old participated in the current study, including 289 males (59.8%) and 194 females (40.2%) (the age of the participants is shown in Figure 1).
The average age of the students was 13.3 ± 1.3 years old. A residence history of 92.7% (434 cases) was more than ten years, and 7.3% (34 cases) was less than ten years old in the high-risk area. The average number of density cases in a family was 5.4 ± 1.4, including at least two and a maximum of 12 cases in a family.
The prevalence of the disease based on observation of parasite ova in urine samples and the prevalence of Schistosomiasis based on ELISA was zero (Table 1).
| Variables | No. Positive | Total (n) |
|---|---|---|
| Antibody test a | 0 | 483 |
| Eggs in the urine filtration | 0 | 483 |
Detection of Antibodies Against Schistosoma haematobium Egg Antigen and the Presence of Parasite Eggs in the Urine Samples of Students Participating in the Study
4.2. History of the Disease Symptoms
The results of the current study show17.2% (81 cases) had a history of burning during urination. However, this rate regarding pain during urination was 7.8% (37 cases). The rate of hematuria in the students was recorded in 1.5% (7 cases) of the participating students in the study, and this was recorded in 11.9% (53 cases) of student family members.
4.3. Prevalence of Risk Factors Associated with Schistosoma haematobium
Results of the present study express that 72.9% (345 and 21.6% (102 cases) of the students had a history of swimming and washing clothes in open water sources such as rivers, respectively; however, 32.5% (153 cases) mentioned a history of contact with open water sources through agricultural activities, and 11.3% (53 cases) had a history of traveling to the endemic country of the disease, Iraq.
5. Discussion
Schistosomiasis is an acute and chronic disease in tropical and subtropical regions. In 2019, about 236 million people needed preventive treatment with praziquantel, and only half of them received the treatment (15). Therefore, this disease still needs to be considered. The aim of this study was to investigate the prevalence of S. haematobium in high-risk areas of this disease north of Khuzestan province. In this study, the prevalence of S. haematobium in students' urine was zero, which is in line with the goals of WHO to control Schistosomiasis.
In a study, S. haematobium was isolated only from Mauritius, and in 1988, the Ministry of Health carried out a special control program.
Health software training was done in the screening program for microhematuria or ova in urine. The prevalence of Schistosomiasis on the island has decreased significantly after applying for the WHO program in the
Mauritius. Amongst the 5- to the 11-year-old school population, the prevalence rate went from 0 to 8.2 percent in 1988 and 1989 to zero in all schools surveyed in 1991. The prevalence in the general population has decreased from an average of 6.6% in 1988 to 0.9% in 1992 (21).
Schistosoma haematobium was endemic in Ancient Egypt. Infection was diagnosed in mummies 3000, 4000, and 5000 years old. S. haematobium was highly prevalent (60%) in the Nile Delta and Nile Valley South of Cairo in districts of perennial irrigation. In 1990, a study conducted in nine governorates of Egypt confirmed the change in the pattern of schistosomiasis transmission in the Delta. There was an overall reduction in S. mansoni prevalence while S. haematobium continued to disappear. All schistosomiasis control projects implemented in Egypt from 1953 to 1985 adopted the transmission control strategy and were based mainly on snail control supplemented by anti-bilharzial chemotherapy (22).
WHO's strategies for schistosomiasis control are based on the broad treatment of at-risk groups, access to safe water, improved hygiene, health education, and snail control, which have been controlled over the past 40 years in several countries, including Brazil, Cambodia, China, Egypt, Mauritius, Islamic Republic of Iran and Saudi Arabia have been successful (15).
Related studies of Schistosomiasis in Iran have been starting since 1949. The investigation results have shown that this infection existed in the villages of Khuzestan province, southwest Iran. The number of infected was estimated to be between 25,000 and 35,000 cases.
The highest infection rate was in people aged 11 to 15 (23). The current serology studies show that the prevalence of S. haematobium infection in students aged 10 - 15 years was zero. These results were in line with our present study conducted by Amarir et al. in 2009 (24).
The results of all S. haematobium medical lab serology tests of children aged 1 - 16 years in Morocco have been declared negative in another study, which indicates the cessation of transmission in the endemic foci of the country after the interventions of WHO to eliminate Schistosomiasis (24).
There is a history of S. haematobium presence in Iran, and its freshwater Bulinus truncatus snail habitat as a host, then all related studies have been conducted in Khuzestan province. According to the latest surveys carried out in Khuzestan province, we have had a relative decrease in the abundance of B. truncatus snails, so we reached 12 thousand snail habitats in 1997 from 17 thousand in 1991, and snail habitats have decreased from 93 places in 2000 to 83 in 2003 (25).
Between 1975 - 1980, 1981 - 1990, and 1991 - 2000, there were 1582, 761, and 79 cases of S. haematobium in the Khuzestan province. In 2001 only one case was reported from Ahvaz; indeed, this was the last case of urogenital Schistosomiasis in Khuzestan and Iran. Prevalence from 1.064% between 1975 and 1980 slumped to 0% in 2012 - 2013 (26).
In Iran, the care of schistosomiasis disease will be carried out based on five strategies, and due to the waste of time and energy, the activities in strategy one (including diagnosis, rapid detection of infection, and timely treatment (passive and active detection of infection) through urine tests - active program when positive cases are reported - monitoring the arrival of cross-border patients - establishing systematic communication and activation of bilharziosis care in border care centers with Iraq) and two (including continuous and regular malacology and habitat control host interface) is performed (25).
In the present study, 72.9% (345 cases) of students had a history of swimming in open water sources such as rivers. 21.6% (102 cases) had a history of washing clothes in open water sources, 32.5% (153 cases) mentioned a history of contact with open water sources through agricultural activities, and 11.3% (53 cases) had a history of traveling to a disease endemic country. They had Iraq.
5.1. Conclusions
Considering that the prevalence of the disease based on the observation of parasite eggs in urine samples and also the prevalence of infection based on the examination of antibodies against S. haematobium in the blood serum of students was zero, indicating the cessation of transmission and elimination of the disease in the investigated area in the north of Khuzestan province.
Because of the snail habitat in the rivers of the studied area and the prevalence of risky behaviors in the local population, disease care needs to be proactive to detect the reappearance of the disease early in the health system.