1. Background
Immune system disorders are one of the main problems of dialysis patients. End-stage renal disease (ESRD) patients undergoing hemodialysis have disorders in both the innate immune system and the adaptive or specific immune system (1, 2). Concerning the innate immune response, defects in the function of neutrophils and the complement system have been reported in these patients (3, 4). Besides, the imbalance of Th1 and Th2 cell responses plays a significant role in specific immune system disorders in hemodialysis patients. This imbalance dysregulates cellular and humoral immune responses (1, 5).
These disorders progress with the duration of kidney disease and dialysis treatment and have a positive association with side effects such as cardiovascular diseases, infections, and cancers. These patients constantly suffer from chronic inflammation, which causes malnourishment, excessive emaciation, and cachexia, further weakening the immune system. This immune system weakness leads to the aggravation of tissue degeneration and premature aging (1). Renal patients undergoing hemodialysis and ESRD are at risk of various infections (4, 5) due to the weakening of the immune system (1). Also, their immune system's response to vaccines is low (6).
Neisseria meningitidis was first discovered by Austrian microbiologist Anton Weichselbaum in 1887 (7). Meningococcus is the bacterium that causes meningitis. This bacterium can also cause meningococcal disease, a life-threatening blood infection (8). This bacterium is thought to be present in the throat of 5 to 15% of adults as normal, non-pathogenic flora. Meningococcus is transmitted through respiratory secretions and saliva, such as sneezing, coughing, and chewing toys. Initial symptoms include fatigue, high fever with neck stiffness, and headache, leading to coma and death if left untreated. The symptoms of meningococcal meningitis are similar to the clinical symptoms of meningitis caused by pneumococcus and Haemophilus influenzae. Meningococcemia (blood infection) is another disease caused by meningococci. The characteristic of this disease is the formation of red rashes that do not change color with pressure. The disease is fatal; 50% of patients will die only a few hours after the onset of symptoms. Other complications include Waterhouse-Friedrichsen syndrome (bilateral adrenal hemorrhage due to fulminant meningococcemia) and disseminated intravascular coagulation (9).
Meningococcal disease is one of the most important causes of death in children in developed countries and causes epidemics in Asia and Africa. Meningococcal meningitis affects one in every 100,000 people in the United States annually. Infection with this bacterium affects all age groups (10). In the Worth 2018 study conducted on hemodialysis patients in Australia, 1% of septicemia causes were reported to be due to meningococci (11).
Immune system disorders are one of the main problems of dialysis patients. Kidney patients undergoing hemodialysis have disorders in both the innate immune system and the acquired or specific immune system (1, 2). The increasing incidence of antibiotic resistance in this bacterium (12) shows the importance of preventing infection with this bacterium.
As meningococcal disease is more dangerous for the elderly and patients with immune system defects, such as kidney patients undergoing hemodialysis (13), vaccination of hemodialysis patients against meningococci seems necessary (14). Since this vaccine is not prescribed to Iranian hemodialysis patients, if there is an antibody against or, in other words, immunity to this bacterium, it can be concluded that this response of the immune system is a result of infection with this bacterium (naturally acquired immunity) (15, 16).
2. Objectives
This research was conducted for the first time to investigate the prevalence of naturally acquired immunity to Meningococcus and its association with the duration of dialysis, gender, and age in hemodialysis patients referred to Jahrom Hemodialysis Center.
3. Methods
3.1. Ethics Statement
Before starting the research, all patients of the research community were asked to complete an informed consent form. The research protocol was approved by the Research Ethics Committee of Jahrom University of Medical Sciences, Iran (IR.JUMS.REC.1400.079).
3.2. Participants
In the present descriptive cross-sectional study, conducted from March to August 2022, the research population consisted of patients suffering from chronic kidney disease undergoing hemodialysis referring to Jahrom Hemodialysis Center. In this study, sampling was done by the census, and all patients (n = 91) were included.
3.3. Demographic Data
Demographic data, such as sex, age, duration of hemodialysis, and the number of hemodialysis sessions per week, were extracted from the patient's medical files and recorded in the questionnaire. No participants had primary or secondary immunodeficiency syndrome, and all had a negative Human Immunodeficiency Virus (HIV) test.
The willingness of the patients to participate in the study was the main criterion for inclusion. Incomplete medical files, primary or secondary immunodeficiency disease, positive acquired immunodeficiency syndrome (AIDS) test, receiving immunosuppressive drugs in the past month, and history of meningococcal vaccination were the exclusion criteria.
3.4. Sampling and Laboratory Tests
First, 3 cc of blood was taken from each patient, and the separated sera were used to detect the amount of serum antibody against meningococcal polysaccharide qualitatively (immunity to Neisseria meningitidis). Patients' sera were kept at -20°C until the ELISA test.
In order to determine the immunity to Meningococcus in the patients, anti-meningococcal polysaccharide antibodies were qualitatively determined in serum samples using the ELISA test with a specialized and commercial kit manufactured by Diapro, Italy (Ref. code: MENG.CE) according to the instructions. The results of the ELISA test were interpreted as follows.
The cut-off value of anti-meningococcal polysaccharide capsule antigen IgG was determined by calculating the mean OD450 nm of the negative control (NC) and then applying the "Cut-off = NC + 0.250" formula. Sera with an OD450 nm lower than the cut-off value were considered not reactive for anti-meningococcal polysaccharide capsule antigen IgG or non-immune to Meningococcus. Sera with an OD450 nm higher than the cut-off value were considered positive for anti-meningococcal polysaccharide capsule antigen IgG or immune to Meningococcus.
3.5. Statistical Analysis
Stata version 14 (Stata Corp LP, College Station, TX) was used to analyze the data. Qualitative data are presented as numbers and percentages, and quantitative data are reported as mean and standard deviation. The chi-square test was used to compare seropositivity based on study variables. Univariate and multivariable logistic regression analyses were used to identify the variables affecting anti-Meningococcus antibody status in hemodialysis patients. A p-value less than 0.05 was considered statistically significant.
4. Results
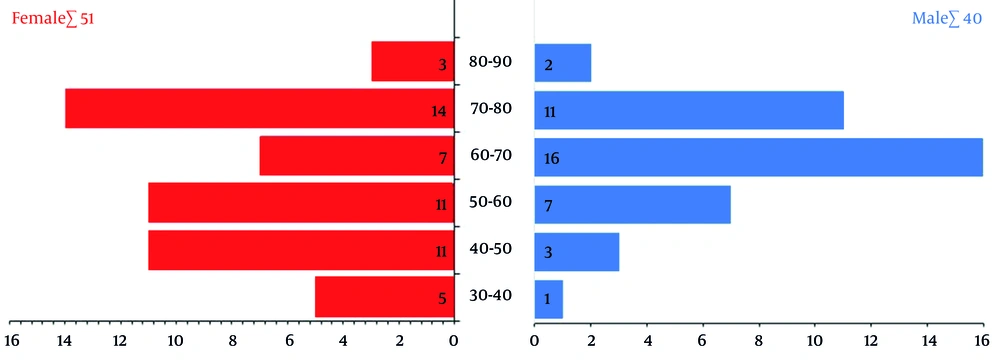
Ninety-one dialysis patients with a mean age of 61.34 ± 13.44 years participated in this study. The average duration of dialysis treatment was 31.70 ± 13.64 months, and the average number of weekly dialysis sessions was 2.85 ± 0.89. Also, 56% of the patients were male, and 78.1% were over 50 years old (Figure 1).
The prevalence of naturally acquired immunity to Meningococcus in hemodialysis patients was 18.68% (95%CI: 10.51 - 26.84%). This prevalence was 30% (95%CI: 17.63 - 46.17) in men and 32.65% (95%CI: 19.20 - 46.10%) in patients with three to five treatment sessions a week (Table 1).
| Categories | Overall Participants (%) | No. Seropositive | %Anti-Meningococcus Immunity (95% CI) | P-Value a |
|---|---|---|---|---|
| Sex | 0.014 | |||
| Male | 40 (44) | 12 | 30 (17.63 - 46.17) | |
| Female | 51 (56) | 5 | 9.80 (4.05 - 21.85) | |
| Age (y) | 0.154 | |||
| 30 - 50 | 20 (22) | 1 | 5 (4.93 - 14.93) | |
| 50 - 70 | 41 (45.1) | 8 | 19.51 (7.06 - 31.96) | |
| > 70 | 30 (33) | 8 | 26.66 (10.35 - 42.98) | |
| Duration of dialysis (mo) | 0.379 | |||
| 6 - 12 | 8 (8.8) | 0 | NA | |
| 12 - 24 | 16 (17.6) | 2 | 12.5 (4.46 - 29.46) | |
| 24 - 36 | 31 (34.1) | 8 | 25.80 (9.93 - 41.67) | |
| 36 - 48 | 30 (33) | 5 | 16.66 (2.91 - 30.41) | |
| > 48 | 6 (6.6) | 2 | 33.33 (8.54 - 75.21) | |
| Number of dialysis sessions per week | < 0.001 | |||
| 1 - 2 | 42 (46.2) | 1 | 2.38 (2.34 - 7.11) | |
| 3 - 5 | 49 (53.8) | 16 | 32.65 (19.20 - 46.10) |
Prevalence of Naturally Acquired Immunity to Meningococcus in Hemodialysis Patients According to Demographic Characteristics
Sex, age, duration of hemodialysis, and the number of weekly dialysis sessions were associated with acquired immunity to Meningococcus. Among them, the number of dialysis sessions per week (OR = 7.32, 95%CI: 2.58 - 20.73, P < 0.001), male gender (OR: 3.94, 95%CI: 1.25 - 12.38, P = 0.019), age (OR = 1.07, 95%CI: 1.02 - 1.12), and duration of dialysis (OR = 1.04, 95%CI: 1.003 - 1.085, P = 0.034), in sequence, were the most potent predictors of acquired immunity to Meningococcus (Table 2).
| Variables | Crude Odds Ratio (OR), OR (95%CI) | P-Value* | Adjusted OR, OR (95%CI) | P-Value** |
|---|---|---|---|---|
| Sex | ||||
| Male | 3.94 (1.25 - 12.38) | 0.019 | 5.43 (1.21 - 24.40) | 0.027 |
| Female | Ref. | NA | Ref. | NA |
| Age (y) | 1.07 (1.02 - 1.12) | 0.006 | NA | NA |
| Duration of dialysis (mo) | 1.04 (1.003 - 1.085) | 0.034 | NA | NA |
| Number of dialysis sessions per week | 7.32 (2.58 - 20.73) | < 0.001 | 8.57 (2.77 - 26.49) | < 0.001 |
Crude and Adjusted Odds Ratios of Study Variables for Seropositivity of Anti-Meningococcus Antibody in Hemodialysis Patients a
In the multivariable model, we entered all the variables mentioned above. Adjusted OR was significantly different in sex and number of dialysis sessions. The chance of seropositivity was the highest in males (OR = 5.43, 95%, CI: 1.21 - 24.40, P = 0.027). In addition, for one unit increase in the number of dialysis sessions per week, the chance of being seropositive increased by 8.57 times (OR = 8.57, 95%CI: 2.77 - 26.49, P < 0.001) (Table 2).
5. Discussion
Immune system disorders are one of the main problems in end-stage renal disease (ESRD) patients undergoing hemodialysis (1, 2), and the extent and severity of these disorders progress with the duration of kidney disease and dialysis treatment (1). The weakening of the immune system in these patients makes them susceptible to various infections (4, 5).
For the first time, this research assessed the prevalence of naturally acquired immunity to Meningococcus and the factors affecting it in hemodialysis patients.
The prevalence of naturally acquired immunity to Meningococcus in hemodialysis patients was 18.68%, including 30% of men and 9.8% of women. Also, 32.65% of patients with three to five weekly treatment sessions had naturally acquired immunity to Meningococcus. Since this research was done for the first time in the world, no information reports the prevalence of naturally acquired immunity in these patients. However, there is information about the prevalence of infection with this bacterium in Iran (but not in hemodialysis patients), which shows the issue's importance.
The prevalence of this bacterium as the causative agent of bacterial meningitis was reported in Iran as 12.78% by Berangi et al. (17), 63.50% by Pormohammad et al. (18), 6.8% by Sadeghi et al. (19), and 13.0% in Houri's et al. in systematic review and meta-analysis (20). In a 2018 study conducted by worth et al. on hemodialysis patients in Australia, 1% of blood infection agents were reported to be meningococcal (11).
The results of the present study are consistent with the results of Rosenstein et al. The research of Rosenstein et al. in 2001 showed that people with a disorder in their immune system (such as those with nephrotic syndrome) are at high risk of developing meningitis (9).
In this present research, we found that the number of dialysis sessions per week, male gender, age, and duration of dialysis treatment, in sequence, were the most potent predictors of acquired immunity to Meningococcus in hemodialysis patients.
The number of dialysis sessions per week, duration of dialysis treatment, and age as factors affecting naturally acquired immunity to meningococci are consistent with the results of previous research in which these factors were associated with the severity of kidney disease and subsequently, the severity of immune system deficiency (1, 4, 5).
However, as no research has investigated the natural immunity to meningococci (immunity that develops without vaccination against infectious agents) in hemodialysis patients, we did not find a justification for the effect of sex (male) on naturally acquired immunity against meningococci.
Regarding the prevention of meningococci infection, vaccines of this bacterium can be used to protect people in the community, especially people with immunodeficiency (14, 21). As a result of Borrow et al.'s study in 2017 in Africa and the Middle East, vaccination is important in preventing meningococcal infection (22).
The limitations of this research can be the small number of investigated patients and the lack of measurement of factors affecting the immune system, such as micronutrient elements (23) in the serum of these patients.
5.1. Conclusions
In conclusion, this research showed that the prevalence of natural immunity against meningococcal is 18.68% of the patients. Considering that kidney patients undergoing hemodialysis are not vaccinated against meningococci in Iran, it can be concluded that 18.68% of the hemodialysis patients examined in this research had a history of meningococcal infection. Considering the susceptibility to this infectious disease and its preventability, it is recommended to vaccinate these patients against meningococcal infection.