1. Background
An epidemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began in China in late 2019, and the global community is currently experiencing the third major outbreak of coronavirus infections. This RNA virus causes an infection that stimulates a significant range of responses, from a complete absence of symptoms to complications related to cytokine storm and acute respiratory distress syndrome (ARDS). Although vaccination is underway worldwide, there is currently no definitive cure for the virus, and we are still witnessing the spreading of infection and deaths from COVID-19 (1). Health sciences aim to discover methods for treating and minimizing the symptoms of diseases. One of the investigated methods was assessing the impact of Vitamin D deficiency on the progression of COVID-19.
Vitamin D is a modulator of expression in about 5% of human genes, most of which are involved in the immune response to pathogens (2). Vitamin D pre-hormone is normally made in the skin when UVB converts 7-dehydrocholesterol in the skin; then, the liver converts 7-dehydrocholesterol to vitamin D (25-hydroxyvitamin D or calcidiol). Calcidiol physiologically is converted to 1, 25-dihydroxy vitamin D or calcitriol in the kidney and all body cells (3, 4). Vitamin D enhances innate cellular immunity by inducing the expression of cathelicidin and defensin genes in monocytes and neutrophils. Cathelicidins kill invasive microorganisms and neutralize the biological activity of their endotoxins by disrupting their cell membranes (5, 6). Additionally, it decreases the production of IFN-γ, TNF-α, and other inflammatory cytokines and raises cytokines generation by Th2 lymphocytes, which causes Th1 cell suppression. Furthermore, it promotes the excitation of T regulatory cells (T Reg) and prevents inflammatory processes.
Vitamin D deficiency has been linked to increased inflammation and an elevated risk of viral infections in the respiratory system, including pneumonia. The risk of tuberculosis, influenza, and respiratory syncytial virus infections is elevated in individuals with vitamin D deficiency, which can negatively impact the outcome of viral infections. Prophylactic vitamin D supplementation can reduce the risk of progression of respiratory tract infections (7-10). Vitamin D deficiency is suggested as a critical risk factor for ARDS that is linked to the fatality rate of SARS-CoV-2 infection in various regions of words (10). Vitamin D deficiency is also associated with increased coagulation complications (11). Thrombosis is common in severe COVID-19 patients (12-14). A low level of vitamin D is frequently observed in older individuals, those who are obese or smoke, and those who have diabetes, hypertension, or gastroenterological conditions. Also, COVID-19 is more prevalent and has more severe complications and mortality rates among the mentioned groups (11, 15).
2. Objectives
Because of the controversial reports of the effect of vitamin D status on COVID-19 patients and disease severity, we evaluated the relationship between vitamin D levels and disease outcomes, including the need for ICU hospitalization and mortality rate in patients with COVID-19.
3. Methods
This cross-sectional study was performed in Ganjavian Hospital, Dezful, affiliated with Dezful University of Medical Sciences, Dezful, Iran. The hospital was a referral center for COVID-19 patients. Using a table of numbers, 50 cases and 50 controls were enrolled in the study after obtaining informed consent. From October 6 to December 30, 2020, patients admitted to the hospital were enrolled in the study by expert specialists using the standard diagnostic protocol of WHO guidance for the disease. We included some demographic and laboratory data such as white blood cell (WBC) count, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), ferritin, white blood cell (WBC) count, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), platelet count, vitamin D (Table 1), and outcomes (i.e., length of hospital stay, discharge, and mortality) (Table 2) that were monitored up to the end of the study. The approval number of the Ethics Committee for the study was IR.DUMS.REC.1400.035. Patients were informed of the purpose of the study, and written/digital consent was taken to participate in the study.
| Variables | Total (n = 100) | Case Group (n = 50) | Control Group (n = 50) | P Value |
|---|---|---|---|---|
| Age (y) | 54.0 (30.0 - 84.0) | 57.5 (31.0 - 84.0) | 52.5 (30.0 - 70.0) | 0.007 |
| WBC (cell/µL) | 6.5 (2.30 - 17.30) | 6.1 (2.30 - 17.30) | 6.6 (4.1 - 11.5) | 0.544 |
| PLT (cell/µL) | 221.0 (73.0 - 774.0) | 206.0 (73.0 - 774.0) | 225.0 (100.0 - 486.0) | 0.560 |
| ALC (cell/µL) | 2.2 (0.1 - 5.7) | 1.1 (0.1 - 4.0) | 3.1 (1.9 - 5.7) | < 0.00001 |
| ANC (cell/µL) | 3.7 (1.3 - 14.8) | 4.4 (1.3 - 14.8) | 3.1 (1.3 - 6.4) | < 0.001 |
| Vitamin D (ng/mL) | 29.0 (8.0 - 77.0) | 29.0 (8.0 - 76.0) | 31.0 (15.0 - 77.0) | 0.366 |
| ≥ 30 (Normal) | 49 (49.0) | 23 (46.0) | 26 (52.0) | 0.602 |
| 20 - 29 (Insufficient) | 31 (31.0) | 15.0 (30.0) | 16 (32.0) | |
| < 20 (Deficient) | 20 (20.0) | 12.0 (24.0) | 8 (16.0) | |
| AST (IU/L) | 23.0 (6.0 - 109.0) | 25.5 (6.0 - 109.0) | 19.0 (11 - 59) | < 0.001 |
| ALT (IU/L) | 21 (4.0 - 103.0) | 27.0 (4.0 - 103.0) | 18.0 (12 - 60) | < 0.001 |
| ALP (IU/L) | 120.0 (24.0 - 954.0) | 181.0 (38.0 - 954.0) | 83.5 (24.0 - 636.0) | < 0.00001 |
| Ferritin (ng/mL) | 150.0 (10.0 - 1116.0) | 328.0 (96.0 - 1116.0) | 86.5 (10.0 - 292.0) | < 0.00001 |
Comparison of Demographic and Clinical Characteristics Between Case and Control Groups a
| Variables | Total (n = 50) | Non-survivor (n = 13) | Survivor (n = 37) | P Value |
|---|---|---|---|---|
| Age (y) | 57.50 (31.0 - 84.0) | 64.0 (41.0 - 84.0) | 52.0 (31.0 - 83.0) | 0.064 |
| WBC (cell/µL) | 6.1 (2.30 - 17.30) | 7.30 (3.0 - 14.9) | 5.9 (2.30 - 17.30) | 0.707 |
| PLT (cell/µL) | 206.0 (73.0 - 774.0) | 221.0 (101.0 - 774.0) | 205.0 (73.0 - 486.0) | 0.825 |
| ALC (cell/µL) | 1.1 (0.1 - 4.0) | 1.0 (0.1 - 2.9) | 1.1 (0.2 - 4.0) | 0.816 |
| ANC (cell/µL) | 4.4 (1.3 - 14.8) | 4.5 (1.6 - 11.7) | 4.3 (1.3 - 14.8) | 0.723 |
| Vitamin D (ng/mL) | 29.0 (8.0 - 76.0) | 18.0 (15.0 - 24.0) | 34.0 (8.0 - 76.0) | < 0.001 |
| ≥ 30 (Normal) | 23 (46.0) | 0 (0.0) | 23 (62.2) | < 0.0001 |
| 20 - 29 (Insufficient) | 15 (30.0) | 5 (38.5) | 10 (27.0) | |
| < 20 (Deficient) | 12 (24.0) | 8 (61.5) | 4 (10.8) | |
| AST (IU/L) | 25.5 (6.0 - 109.0) | 26.0 (14.0 - 85.0) | 25.0 (6.0 - 109.0) | 0.535 |
| ALT (IU/L) | 24.0 (4.0 - 90.0) | 27.2 (12.0 - 72.0) | 23.0 (5.0 - 106.0) | |
| ALP (IU/L) | 181.0 (38.0 - 954.0) | 218.0 (42.0 - 954.0) | 180.0 (38.0 - 636.0) | 0.278 |
| Ferritin (ng/mL) | 328.0 (96.0 - 1116.0) | 422.0 (96.0 - 1116.0) | 264.0 (124.0 - 1003.0) | 0.177 |
Demographic and Clinical Findings of COVID-19 Patients Based on In-hospital Mortality Status a
Eligibility criteria for sample selection were confirmed chest computed tomographic (CT) scan, X-ray, and/or positive RT PCR for SARS-CoV-2 infection and no history of cardiac, kidney, and liver diseases. The CBC samples were collected using EDTA as an anticoagulant. Additionally, clot samples were collected from both patients and controls. The separated sera were then stored at -70ºC. The CBC tests were done within 3 hours of sampling. The serological and biochemical parameters were performed on frozen samples with the usual measurement methods in clinical medical diagnostic laboratories. Vitamin D levels were measured using ELISA, and liver function tests were conducted using Man company's enzymatic kits.
3.1. Statistical Analysis
Quantitative data are presented as median and range, and qualitative data as count (percentage). The Shapiro-Wilk test was performed to assess the normality of data distribution. To compare clinical and demographic variables between the studied groups, the Mann-Whitney U and t-tests were employed. The chi-square test and Fisher's exact test were used to compare the levels of vitamin D (normal, insufficient, and deficient) across the groups. Univariate logistic regression assessed the association between vitamin D and in-hospital mortality status. The Hosmer-Leme show test was carried out to check the model's goodness of fit. All analyses were performed using SPSS at a P-value < 0.05 as statistically significant.
4. Results
The mean age of the patients was 57.50 (31.0 - 84.0), with 19 (38%) in the 30 - 50 age group and 31 (62%) above 50 years old. Out of the total sample size, 28 (56%) were male, and 22 (44%) were female. This study revealed a significant difference in AST, ALP, ALT, ANC, ALC, and ferritin levels but no statistically significant difference in vitamin D3 status between the case and control groups. No statistically significant differences were observed in hematologic, biochemical, and serologic parameters between the survivor and non-survivor groups, except for vitamin D. In this study, 36% (n = 18) of patients were transferred to the intensive care unit (ICU). Among non-survivors, 38.5% (n = 5) had vitamin D insufficiency, and 61.5% (n = 8) had vitamin D deficiency. The mortality rate in the group with vitamin D deficiency and insufficiency was 26% (13). The mean age of survivors and non-survivors differed significantly, with survivors having a mean age of 52.0 years (range: 31.0 - 83.0) and non-survivors having a mean age of 64.0 years (range: 41.0 - 84.0) (P < 0.001) (Tables 1 and 2). The insufficiency and deficiency of vitamin D3, as well as elevated levels of AST, ALT, ALP, and ferritin, which had correlations of -0.374, -0.300, -0.131, and -0.137, respectively, were found to be associated with longer hospitalization times (Table 3).
| Laboratory Variables | Vitamin D | WBC | PLT | ALC | ANC | AST | ALT | ALP | Ferritin |
|---|---|---|---|---|---|---|---|---|---|
| Correlation | - 0.374 | 0.172 | 0.038 | 0.060 | 0.157 | - 0.300 | - 0.131 | 0.280 | - 0.137 |
| P-value | 0.008 | 0.232 | 0.791 | 0.681 | 0.276 | 0.034 | 0.364 | 0.049 | 0.344 |
Correlation of Laboratory Variables with Hospital Length of Stay in COVID-19 Patients
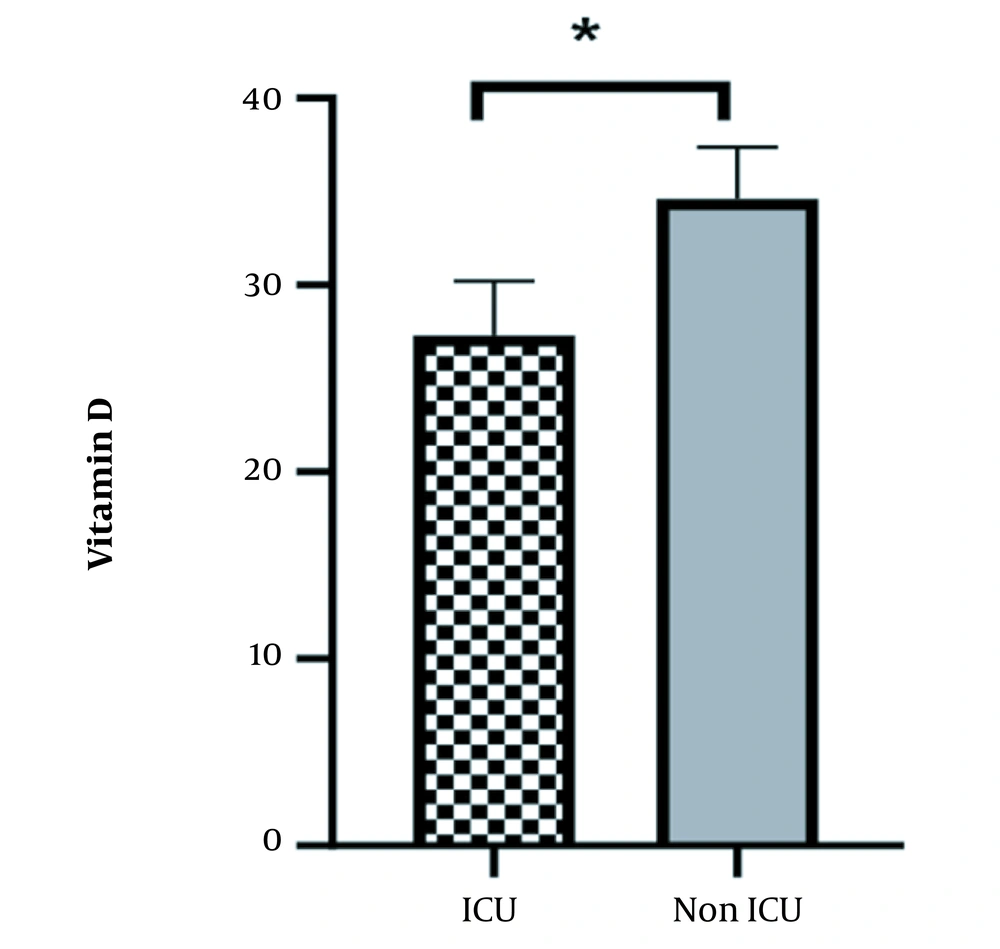
Additionally, ICU admission and death occurred more frequently in the group with lower vitamin D levels compared to the group with sufficient levels (P < 0.05) (Figure 1). In the univariate logistic analysis, vitamin D levels in COVID-19 patients were analyzed as a continuous variable and found to be significantly associated with a decreased risk of in-hospital mortality (OR = 0.79; 95% CI, 0.68 to 0.91; P = 0.002). The Hosmer-Lemeshow test showed that the logistic regression model fits well with the data (P-values > 0.05) (Table 4).
5. Discussion
We evaluated the relationship between vitamin D levels and disease outcomes, including the need for ICU hospitalization and the mortality rate in patients with COVID-19. The results showed that vitamin D deficiency was associated with higher ICU admission and mortality. Based on the available literature, vitamin D has an important immunomodulatory function and reduces inflammatory responses. In addition, vitamin D has a role in the production of some antimicrobial peptides, such as defensins and cathelicidin, and thus can play a role against pathogens (16, 17). There is some evidence of the role of vitamin D in the regulation of synthesis and secretion of anti-inflammatory factors and cytokines like interleukin (IL-4), IL-10, and transforming growth factor (TGF-β) (7, 9, 10). Recent research has shown that vitamin D insufficiency and deficiency are the most important factors in susceptibility to respiratory system infections and might contribute to COVID-19 severity and mortality rates (18-20). In contrast, in other research, no significant relationship was reported between vitamin D levels and the risk of COVID-19 infection (21). According to the present study, vitamin D deficiency affects the death and ICU entrance of patients. We suggest that vitamin D supplement therapy in SARS CoV 2 infection may ameliorate the severity and progression of COVID-19 infection.
5.1. Conclusions
It is essential to recommend vitamin D supplements that are safe and cheap to attenuate the COVID-19 severity.