1. Introduction
Several anti-malarial strategies were employed to fight malaria between 1957 - 1980 in Iran, and, as a result, the northern part of the country was protected against this infection, but the southern part continued to face the health challenge (1). The mosquito control program was initiated by using DDT insecticide, but it was replaced with other control methods such as mechanical/biological methods for larvae, space spraying, application of insecticide-treated nets (ITNs), indoor residual spraying (IRS), and application of repellents (2). Needless to say, the exclusive and widespread application of pesticides led to the resistance of anopheles to them (3). The national malaria program shifted from the control phase to the elimination in 2010 (4, 5). At present, this parasitic infection is confined to Hormozgan, Kerman, Sistan and Baluchistan provinces, located in southern Iran (6). Out of these provinces, Sistan and Baluchestan was more affected by the disease, so the incidence rates in four years (i.e., between 2011 - 2014) were 89.94, 43.9, 38.3, and 30.66 per 100000 people, respectively. The highest numbers of malaria cases were reported from cities including Sarbaz, Nickshahr, and Chabahar in Sistan and Baluchestan province as well as Bandar Abbas, Bandar Jask, and Bandar Lengeh in Hormozgan province (7). Moreover, vivax malaria was most prevalent in Iranian cases, while falciparum was usually seen in migrant workers from Pakistan and Afghanistan (8, 9). In general, no local malaria cases have been reported from Iran and Hormozgan province since 2018, and the malaria elimination program has been successfully managed (10). Currently, imported cases from neighboring countries are a problematic issue, so a quarter of all cases in the Eastern Mediterranean Region are mostly from Afghanistan and Pakistan (11). Furthermore, the coronavirus disease (COVID-19) has become a worldwide pandemic and health disaster. As of June 2023, according to World Health Organization (WHO), a total of 767,750,853 confirmed cases worldwide - including 6,941,095 deaths - were reported, and a total number of 13,381,641,358 vaccine doses were administered (12). The total of confirmed coronavirus cases in our province was 49,757, including 41,990 outpatients, 6,711 hospitalized patients, and 1,044 death. According to the unpublished data, the current population of Hormozgan is 2,036,363. Although the indigenous malaria cases have declined dramatically for 20 years (i.e., between 2000 to 2020), the COVID pandemic has continued to affect the programs designed to combat malaria, particularly in those countries where hydroxychloroquine and chloroquine have been used as medications for treating COVID. The effectiveness of these compounds against Coronavirus has not been confirmed (13, 14). This study aimed to report two malaria cases misdiagnosed as COVID-19 patients in Hormozgan province and to assess the effects of COVID-19 on these cases from all aspects (i.e., case-finding, diagnosis, and treatment).
2. Case Presentation
2.1. First Case
A 24 years old man from Badakhshan province in northeastern Afghanistan, near the border with Tajikistan, left his province on February 20, 2021, and arrived in Nimruz province, southwestern Afghanistan, after ten days. Nimruz province borders Iran and Pakistan; therefore, he spent two days crossing Pakistan and entering Iran illegally through Sistan and Baluchestan province. On his path to his final destination, he had to pass the desert and unknown areas. He crossed Kerman and Isfahan provinces into the capital city of Tehran and then found a job in a technical engineering office and worked there for five months and ten days. On August 1, 2021, he felt sick with a high fever and shaking chills and, therefore, was referred to a hospital. He underwent a COVID-19 test with a swab by an on-call physician, but the result was negative. It is noteworthy that he took chloroquine on a voluntary basis only for one day before receiving the result. The next day, on August 3, 2021, he was referred to the general practitioner of the same hospital again. Prescription drugs included: Pantoprazole 40 mg (ampoule), apotel 1 gr (ampoule), sodium chloride 0.9% 500 mL, codimal-extra (tablet), captopril 25 mg (tablet), bromhexine (syrup), and mocozift (syrup). The patient also received injections of neurobion and vitamin B complex. On August 8, he complained about the symptoms persisting for 28 days, the ineffectiveness of the prescribed drugs, and the worsening of his condition. The patient continued to suffer from the symptoms for a month. Afterward, he moved to the tourist island of Kish through the Bandar Abbas-Charak route on September 1 for labor and settled in a building with suitable amenities such as electricity and an air conditioner. However, he was unable to work for about 10 days. On September 3, he suffered from a fever reoccurring every other day, body aches, and severe pain. To relieve his pain, the on-call physician of Kish Hospital prescribed some drugs, including multivitamins (capsule), prednisolone (amp), and ketorolac 30 mg (amp). On September 7, he was referred to the Health and Control Disease Center in Kish and underwent several tests except for a malaria test. His biochemistry lab findings were normal (e.g., fasting blood sugar (FBS), blood urea nitrogen (BUN), creatinine (Cr), Cholesterol (including total chol.-LDL and HDL), and triglyceride (TG)). Also, liver function test (LFT) included AST-ALT, and alkaline phosphatase was normal with no change. On the contrary, C-reactive protein (CRP), an acute phase reactant factor, was positive (1+). Erythrocyte sedimentary rate (ESR), which rises in inflammatory and febrile situations, was equal to 125. Complete blood count (CBC) test showed a decreased counting of red blood cell (RBC) with a Hb level equal to 7.6 and a hematocrit percentage equal to 24%. Furthermore, a decreased platelet count (i.e., thrombocytopenia) was seen since his platelet count range was 107 mL. It should be noted that the normal range of platelet count is between 150 to 450 mL. Therefore, the patient was treated symptomatically, and on September 16, he was recommended to undergo a COVID-19 test. Finally, he was referred to a private physician and was recommended to have a blood test at Kish Hospital. Erythrocyte sedimentary rate was raised to 95. Complete blood count-differential (CBC-diff) demonstrated a decreased RBC count and hematocrit (HCT) percentage with severe anemia (hemoglobin amount 8.6 g/dL), but the differential count of lymphocyte, neutrophil, etc. was normal. Kish Medical Laboratory informed the Health Center about the positive vivax malaria of our case on September 21 at 8 pm. All blood-stage of the vivax parasites (i.e., gametocyte, schizont, trophozoites) were seen in the blood smear during microscopic examination. Our approved malaria case was a reminder of the sad fact that how someone may waste a lot of money (forty million Iranian Rials or ~ US$150, in this case) on medical treatment, although s/he could be treated almost for free.
2.2. Second Case
A 46 years old man set off on foot with his family and a relative from Quetta, a city in Pakistan bordering Iran, on September 10, 2020, and arrived in Mirjaveh, Iran, on September 12. Then this Pakistani man moved to Zahedan on September 13 and then to Bandar Abbas (the capital of Hormozgan province) on September 14, 2020. He stayed in a village near Bandar Lengeh named Divan and worked there as a fish seller, but his relative resided in Kong city, 40 km distance from Divan village. Both of them accessed their favorite amenities in the rented houses, such as electricity and cooling equipment. In our considered case, the 46 years old man complained of a fever with unknown origin (FUO), left flank pain, dysuria, myalgia, and a non-productive cough on September 17 and, therefore, was admitted to rule out the Urosepsis and COVID-19 diagnoses. O2 (O2 saturation) was 96%. A physical examination of his lungs was carried out, according to which the auscultation was clear, with no abnormal breath sounds. Potassium (K) was 3.3 mEq, Plt 760 mL in biochemistry and mean corpuscular volume (MCV) in hematology of 79.7 fl. Protein was positive in urine. Serum glutamic-oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) enzymes were 65 U/L land 104 U/L, respectively. Chest CT scan showed no evidence of patchy ground-glass opacity (G.G.O) in lung parenchyma, which may have been due to the early stage of COVID-19. Lung parenchyma, trachea, and bronchus were intact. Pulmonary hilar structures, trachea, and esophagus were all normal. His pericardium had normal thickness without evidence of thickening, calcification, or effusion. No pleural effusion was seen. His chest wall, including ribs, was normal, with no evidence of bone destruction. The findings from the CT scan of his abdomen and pelvic were normal. The initial treatment included sodium chloride 0.9%/2 liters/IV infusion during 24 hours, ampule ciprofloxacin 400 mg/IV per 12 h (intravenous), ampule apotel 1 gr/IV when the temperature was above 38.5 degrees, and pantoprazole 40 mg/PO (tablet) on a daily basis. The following drugs were then added to our patient treatment after a consultation with a cardiologist: Ampule enoxaparin 60 mg/SC/BID and atorvastatin 20 mg/daily (tablet). On September 18, a PCR test was taken from him, and the suspected COVID-19 result was negative. Moreover, CRP was high in his blood (equaled 44 mg/L). The internist prescribed these medicines: Syrup bromhexin/10 cc/ q 8 h, nebulizer pulmicort/BID, and nebulizer duolin/TDS. According to the progress note, his symptoms were persistent to a great extent, and his blood pressure was within the normal range. The patient was first feverless but then developed fever and nausea. O2 saturation was normal during this period without supplemental oxygen therapy. On September 19, favipiravir 200 mg (antiviral treatment. Furthermore, the patient was initially given an analgesic (naproxen) based on the clinical and laboratory findings suggesting the presence of COVID-19, but he was discharged on September 21 and recommended to visit an internist later. On October 31, 2020, the patient contracted fever and chills and was, therefore, referred to the health office of Divan village. The next day, he was correctly diagnosed with a vivax malaria case performing malaria rapid diagnostic test (RDTs). He was treated with chloroquine and primaquine based on the national treatment protocol. At the time of the detection, his average temperature was 27°C with humidity of 75%, but no mosquitoes were found or captured in the vicinity of his place. The patient had traveled to Pakistan frequently and illegally.
3. Discussion
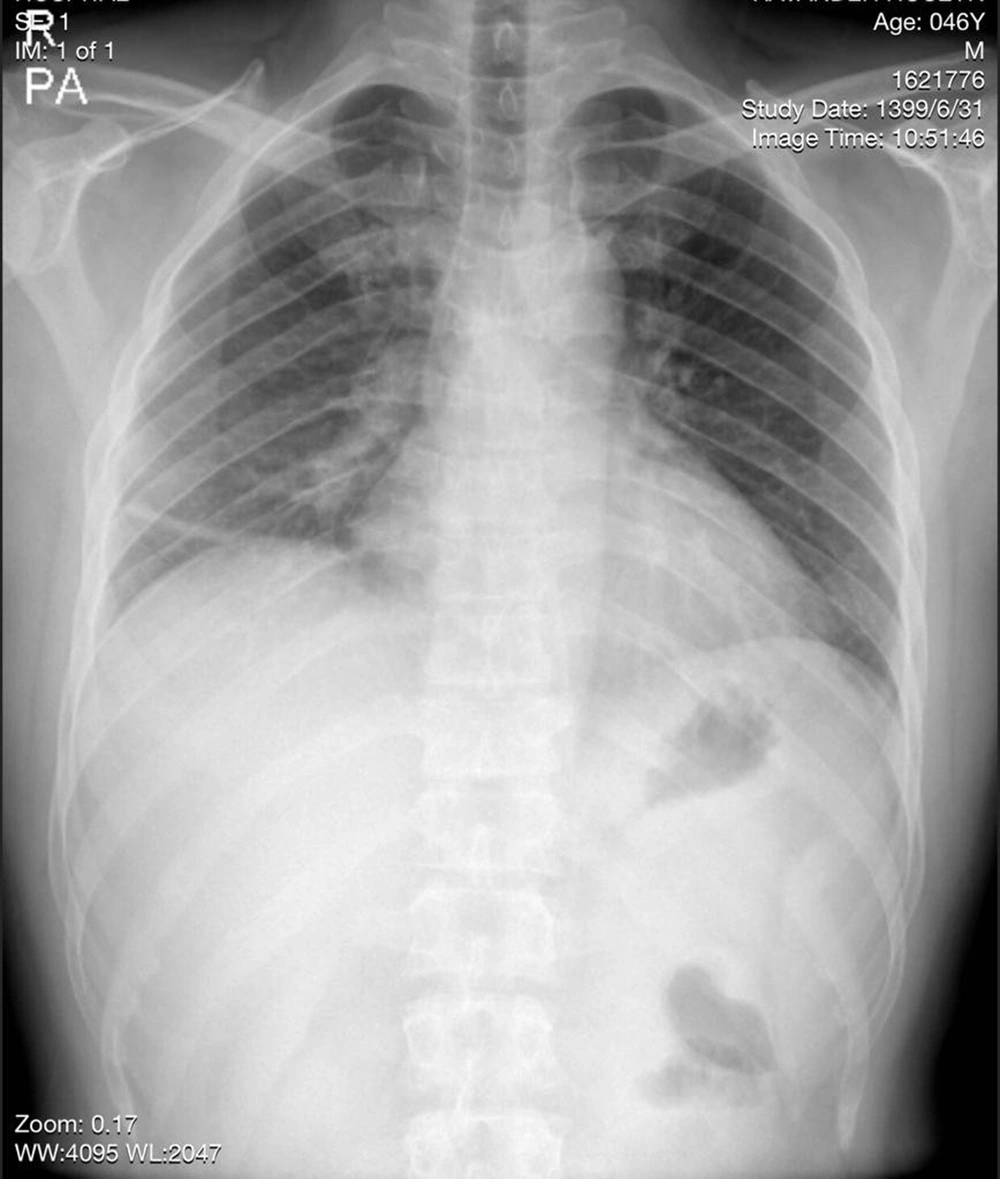
Both cases presented with common symptoms such as fever and shaking chills. In addition, CRP markers may have diverted the doctors' attention to the presence of an infection like that of malaria. The oxygen level and CT image of the second case were normal, and no alternative diagnosis was reached based on his CT scan findings. However, he was treated as a COVID-19 patient based on the clinical manifestations, although some common clinical symptoms (e.g., fatigue, expectoration, and chest tightness) were not observed in him (Figure 1). Some asymptomatic COVID-19 cases have normal chest CT, which makes it difficult to treat a suspected case as a confirmed COVID patient (15). Some medical staff argue that they should start treatment even if there are no signs of COVID. On the contrary, our patients were from the malarious area where malaria was locally transmitted, but the physicians failed to consider the epidemiological criterion of malaria in these endemic sites. A great number of immigrants entered Iran illegally from the neighboring countries, but Iran's health care system is mostly unable to trace them because they usually adopt different methods to conceal their identity. A peak of COVID, with a total of 10,789 confirmed coronavirus cases, was recorded in Lengeh around August and March 2020. Furthermore, the COVID-19 pandemic put great strain and stress on clinical staff and caused reducing the number of personnel due to the risk of infection or mortality, which shifted their focus onto, especially outpatients and suspected cases. However, a delayed diagnosis or treatment was not the case with the health care providers working at health offices in smaller areas or villages since they had lower workloads. This may have explained the reason why our second patient was quickly diagnosed with malaria in a village. Our cases - particularly the second case staying with his family members - had been in close contact with people since their arrival in Kish Island and Bandar Lengeh city until the diagnosis time. Although no indigenous malaria cases had been reported from Kish since 2007, and all foci were clear, some vector activities were observed. It is noteworthy that Kish Island is a tourist destination and an important economic zone in southern Iran with a lot of annual visitors. Recorded malaria cases had been imported from adjoining countries into this district. The population is extremely fluid. Traders or unskilled workers move there and often leave to find a job. Bandar Lengeh is a district with a high potential for malaria transmission. The last indigenous case was recorded in 2008 from Bandar Lengeh. The introduced cases were announced in 2017, then imported cases from Afghanistan and Pakistan took over up to now in Lengeh. Two cases were detected passively. The first patient was referred to a hospital, and the second one was referred to a health office in a village. The COVID-19 pandemic affected the active case detection of malaria among high-risk groups performed by health care that were involved in the case finding routinely. Moreover, these personnel participated in the COVID-19 sampling process because of a shortage of staff. Misdiagnosis of our cases placed enormous financial burdens on them due to the hospitalization and undergoing several tests, while anti-malarial drugs were free of charge, and they could have been easily treated almost for free. They had no sustainable sources of income, and their medical care was not covered by health insurance. In addition, the possible side effects of the coronavirus drugs may have posed serious and long-term risks to these patients who had left their countries in the hope of a better life. Another part of misdiagnosis was that they were desperately wandering from home to clinics and vice versa. The first case took CQ for only a day by his own decision. CQ should be taken with primaquine for Plasmodium vivax. If he continued with CQ monotherapy, it would cause relapse or resistance as uncomplicated treatment (16, 17). In Iran, CQ is prescribed for those who are exposed to coronavirus, particularly for outpatients or those in the early stages of infection. Therefore, misdiagnosis in suspected COVID cases may happen. A suspected coronavirus patient receives CQ, while a malaria patient needs CQ and primaquine in combination with its own special does (18). If the case is falciparum malaria, the situation is likely to worsen because falciparum is more resistant to CQ and provides a recurrence of parasitemia (19). Consequently, COVID-19 delays malaria prevention measures and increases the exposure of health providers to coronaviruses, and minimizes the early seeking of medical assistance (20). Moreover, it can certainly impede malaria management, thereby increasing the rate of malaria incidence (21). World Health Organization global malaria programme is aimed at mitigating the negative impact of the coronavirus in malaria-affected countries during the time of the evolving COVID-19 pandemic (22). Fortunately, no local case was observed. However, a new cycle of malaria transmission may begin and continue to an uncontrollable phase so, which spoils the valuable achievement and changes the pattern of malaria elimination programs.