1. Background
Infertility is a major health issue that affects approximately one-third of couples and has recently been on the rise (1). Multiple environmental and acquired factors can influence and cause infertility, with the prevalence of these factors varying across different regions. In men, reproductive status is strongly affected by environmental factors, particularly in the workplace (2, 3). Decreased sperm count, low sperm motility, and sperm deformation are the most significant factors associated with male infertility (4). Low sperm count, known as oligospermia, occurs when the number of sperm per milliliter of semen is less than 15 million. Oligospermia can impair natural fertility in men (5). Issues such as low sperm count and sperm quality problems are highly common in the general population (6).
Sperm motility is another important factor influencing infertility, and it is a key indicator of sperm quality (7). Infertility due to inactivity or poor sperm motility is a common reason for referrals to infertility treatment centers. While several factors may affect sperm motility, many remain unknown in numerous cases. Oxidative stress is intrinsically involved in sexual dysfunction and decreased sperm motility, drawing the attention of researchers (8). Sperm are highly susceptible to oxidative stress due to their cell membranes being rich in unsaturated fatty acids, making them prone to oxygen-induced damage and lipid peroxidation (9).
The primary cause of oxidative stress is the overproduction of reactive oxygen species (ROS), which are known to damage cells and tissues (8). Oxidative stress occurs when ROS production exceeds the body's natural antioxidant defense mechanisms, causing damage to compounds such as lipids, proteins, and DNA. Evidence suggests that infertile men have higher levels of ROS compared to those with normal fertility (10). Elevated ROS levels in sperm can lead to sperm dysfunction, damage to sperm DNA, and reduced reproductive capacity in men (11). Furthermore, mature sperm lack cytoplasmic enzymes and antioxidant defense mechanisms due to the loss of a significant portion of their cytoplasm during the spermatogenesis process (12).
In contrast to oxidative damage, seminal plasma is a rich source of enzymatic and non-enzymatic antioxidants, collectively known as total antioxidant capacity (TAC). These antioxidants, each with specific mechanisms, play a crucial role in protecting spermatozoa against ROS attacks and neutralizing free radicals (13, 14). High levels of ROS in semen, an imbalance between ROS production and decomposition, and a weakened antioxidant system can lead to sperm dysfunction and ultimately sperm cell death (15). Total antioxidant capacity is measured by assessing serum levels of malondialdehyde (MDA), a key indicator of oxidative stress in semen. Evidence has shown higher levels of MDA in infertile men (16).
Several studies have emphasized the importance of a controlled increase in semen antioxidants to enhance sperm quality and motility. Royal jelly, a product produced in the stomach of worker bees through the partial digestion of honey, has been recognized for its potential benefits. This substance, secreted from the submandibular and maxillary glands of bees to feed the queen bee, is a milky white gelatinous substance with a pungent odor, fruity taste, and high nutritional value (4). Royal jelly contains pantothenic acid (B5), pyridoxine (B6), acetylcholine, vitamins A, C, D, and B, as well as mineral salts (K, Ca, Na, Zn, Fe, Cu, Mn), enzymes, hormones, 29 amino acids, and antibiotic compounds (11). It has long been used as a fertility stimulant (13).
2. Objectives
This study aimed to investigate the effects of directly adding royal jelly to frozen-thawed semen and its subsequent incubation on sperm activity and motility. Additionally, we assessed the changes in the TAC of semen following royal jelly treatment.
3. Methods
This interventional study was conducted after obtaining the necessary approval from the Ethics Committee of Dezful University of Medical Sciences in Dezful, Iran (code: IR.DUMS.REC.1398.051). Sixty men who visited the Dezful Infertility Center provided written informed consent to participate in the research. The subjects met the World Health Organization (WHO, 2010) inclusion criteria, which specified normal sperm parameters: More than 15 million sperm per 1 mL of ejaculation, 32% progressive motility, and 4% morphology.
Semen samples were collected from the sixty patients (via self-ejaculation) into sterile containers. Importantly, the patients had abstained from sexual activity for 3 - 5 days prior. Each sample was divided into three parts, corresponding to three groups, including two groups of frozen sperm (to reduce sperm motility under the same conditions) and a control group (fresh sperm).
After one month, one capsule of royal jelly (manufactured by Shahdineh Company, Iran) was added to two batches of the thawed sperm. Each pure royal jelly capsule contained 1000 mg and was dissolved in 10 mL of double distilled water to achieve a concentration of 100 mg/mL. Upon the patients’ arrival, fresh sperm samples were examined using a neobar slide and a counter device to assess the number, motility (including the percentage of progressive/non-progressive sperm and in-situ motility), and morphology (shape and size). For this analysis, the sperm were randomly examined, and the results were averaged from several microscopic fields. Papanicolaou staining was applied to evaluate sperm morphology, and Eosin staining (0.5% in saline) was used to differentiate live, motile sperm from dead or immobile sperm.
To separate the seminal plasma from the sperm-containing portion, the samples were first centrifuged at 2,000 rpm for 10 minutes. The supernatant was then separated and stored for further testing until the samples were aggregated. The MDA level and TAC were measured in the separated seminal plasma sample (1, 2). Total antioxidant capacity was assessed using the ferric reducing antioxidant power (FRAP) method. In this method, under acidic pH created by an acetate buffer, ferric ions (Fe3+) in the Fe3+-TPTZ complex are reduced to ferrous ions (Fe2+), producing a blue color. This color was measured spectrophotometrically at a wavelength of 593 nanometers using a spectrophotometer (Biochrom, UK).
To determine the level of lipid peroxidation, MDA production was assessed as an indicator of this process in the serum samples. This was based on its reaction with thiobarbituric acid, which produces a colored product with maximum light absorption at 532 nanometers. Malondialdehyde levels were calculated using a standard calibration curve and expressed in nanomoles per milligram of protein.
Malondialdehyde was evaluated using a UV-VIS spectrophotometer through spectrophotometric measurements. After removal from the nitrogen tank, the frozen samples were stored at room temperature (20 - 24°C) until thawed. Royal jelly was then added to one group of the thawed semen at a concentration of 0.5% of the semen volume and incubated at 37°C for three hours. The other group of thawed samples was incubated for three hours without royal jelly. After the incubation period, the sperm samples were analyzed, and all the parameters evaluated in the fresh sperm group were reassessed.
3.1. Statistical Analysis
All analyses were conducted using SPSS version 22 (SPSS Inc., Chicago, IL, USA). Group variances were assessed through one-way analysis of variance (ANOVA). Fisher’s least significant difference (LSD) test was applied to evaluate significant differences between the groups. A P-value of ≤ 0.05 was considered statistically significant.
4. Results
In total, semen samples were collected from 60 men referred to the infertility center, and each semen sample was equally divided into three groups. The control group consisted of fresh sperm, while the two intervention groups included frozen and then thawed sperm. In one of the intervention groups, royal jelly was added to the semen samples after thawing. Semen analysis was performed on all study groups to evaluate sperm immotility, progressive sperm motility, non-progressive sperm motility, and sperm morphology (Table 1).
| Parameters and Groups | Sperm Immobility | Non-progressive Sperm Motility | Progressive Sperm Motility | Sperm Morphology |
|---|---|---|---|---|
| Fresh sperm | 36.4 ± 3.19 C | 10.3 ± 2.77 A | 52.4 ± 4.26 A | 12.3 ± 3.27 A |
| Thawed sperm with royal jelly | 56.3 ± 4.75 B | 12.3 ± 3.41 A | 39.3 ± 3.41 B | 11.12 ± 3.5 A |
| Thawed sperm without royal jelly | 62.6 ± 4.16 A | 13.3 ± 3.16 A | 21.4 ± 3.17 C | 10.4 ± 3.21 A |
| P-value | 0.009 | 0.06 | 0.01 | 0.07 |
a Each capital letters in each column indicate a significant difference at P ≤ 0.05.
The comparison of sperm morphology between the study groups indicated no significant difference. Additionally, no significant difference was observed in sperm morphology between the fresh semen group, the semen group after thawing with royal jelly, and the semen group after thawing without royal jelly.
The results of ANOVA showed a significant difference between the study groups in terms of immotile sperm (P = 0.042). Furthermore, the assessment of the mean immotile sperm indicated a difference between the fresh sperm group, the frozen sperm group with royal jelly, and the frozen sperm group without royal jelly. The lowest level of immotile sperm was observed in the fresh semen group (mean: 36.82 ± 4.19). The number of immotile sperm increased after the freezing process, while the intervention group with added royal jelly after thawing had a lower mean immotility (mean: 56.84 ± 3.75) compared to the group without royal jelly (mean: 62.4 ± 3.16), indicating the improvement caused by the addition of royal jelly.
The comparison of progressive sperm motility between the three study groups indicated a significant difference in terms of progressive motility (P = 0.034). Although freezing reduced motility, the mean motility in the group with added royal jelly was significantly higher (mean: 39.76 ± 3.41) compared to the group without royal jelly (mean: 21.36 ± 2.17; P < 0.05).
The comparison of non-progressive sperm motility between the three study groups showed no significant difference in non-progressive sperm motility (P = 0.414).
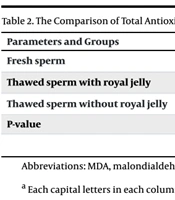
Table 2 shows the comparison of TAC and MDA between the study groups. To compare the means between the three groups, we used ANOVA with a 95% confidence interval and an error rate of 0.05. Significant differences were observed between the study groups in terms of TAC and MDA (P < 0.05). Furthermore, there was a difference in the mean TAC between the fresh sperm group, the frozen-thawed sperm group with royal jelly, and the frozen-thawed sperm group without royal jelly.
| Parameters and Groups | MDA | TAC |
|---|---|---|
| Fresh sperm | 16.4 ± 2.16 B | 1120.6 ± 3.43 A |
| Thawed sperm with royal jelly | 21.3 ± 2.75 A | 947.5 ± 4.38 B |
| Thawed sperm without royal jelly | 24.5 ± 3.12 A | 803.5 ± 4.32 C |
| P-value | 0.004 | 0.008 |
Abbreviations: MDA, malondialdehyde; TAC, total antioxidant capacity.
a Each capital letters in each column indicate a significant difference at P ≤ 0.05.
According to the results, the highest TAC was in the fresh sperm group (mean: 1120.27 ± 3.41), which was significantly higher compared to the other two groups. Moreover, the mean TAC of the frozen-thawed sperm group with added royal jelly (mean: 947.29 ± 4.38) significantly increased compared to the frozen-thawed sperm group without royal jelly (mean: 803.37 ± 4.32) (P < 0.05). A significant difference was also observed in the mean MDA levels of the fresh sperm group (mean: 16.91 ± 2.16) compared to the frozen-thawed sperm group with royal jelly (mean: 21.48 ± 2.75) and the frozen-thawed sperm group without royal jelly (mean: 24.12 ± 3.12). However, no significant difference was noted in the mean MDA between the frozen-thawed sperm group with royal jelly and the frozen-thawed sperm group without royal jelly.
5. Discussion
Sperm quality is considered a significant factor in infertility and is defined by a set of physical characteristics, including sperm motility, each of which affects fertility. In addition to age, these sperm characteristics are influenced by deficiencies in specific substances, vitamins, and antioxidants (9). The antioxidants in semen act as protective agents for sperm. Oxidative stress is a major factor in sexual dysfunction and decreased sperm motility, which has recently attracted researchers' attention (8).
Sperm are particularly prone to oxidative stress due to the properties of their membranes, which are rich in unsaturated fatty acids and therefore more susceptible to oxygen-induced damage and lipid peroxidation (9). Controlling the semen environment is crucial for maintaining sperm health. In some cases of infertility treatment, it becomes necessary to freeze semen temporarily. However, thawing and freezing, as indicated by previous studies, may affect semen quality by reducing sperm motility. Researchers have explored the effects of various materials, interventions, and additive drugs/supplements on semen and sperm quality. In the case of royal jelly, there is a lack of definitive scientific evidence, although some findings suggest that it may effectively treat certain infertile men when consumed orally.
In the current research, royal jelly was found to enhance sperm motility, sperm morphology, TAC, MDA, and ROS. To ensure consistency in evaluating sperm motility, frozen and thawed normospermia samples were used, and the motility variable was only influenced by the freezing and thawing process across all samples.
According to the present study, royal jelly reduced the number of immotile sperm, and the mean immotility was lower in the fresh semen samples compared to those with added royal jelly, which serves as a rich source of antioxidants. The difference in this regard was considered significant. This finding aligns with the results of Nair et al., where the addition of vitamin E (an antioxidant) to sperm increased mean sperm motility compared to the group without added vitamin E (17).
In the current research, another observed effect of adding royal jelly to sperm was on sperm morphology. In a similar study, Peivandi et al. examined the effect of royal jelly on ram sperm morphology and reported a significant difference in sperm shape (18). Additionally, freezing and thawing processes affected both the motility and morphology of sperm in that study, with differences observed when comparing thawed sperm to fresh sperm samples (19). In the present study, although freezing, thawing, and the addition of royal jelly were performed, no significant difference in sperm morphology was detected. In a study by Shirzad et al. (as cited by Abdelnour et al.), human sperm was stored in a nitrogen tank for 40 days, and after thawing, it was found that only a small number of the sperm remained motile. Since freezing sperm by inducing cold shocks diminishes acrosome function, membrane integrity, and sperm motility, the semen samples in the present study were frozen for two weeks and then thawed to decrease sperm motility (19). The results of this research indicated that incubating thawed semen with royal jelly significantly mitigated the effects of the induced cold shock.
In a clinical trial, Shahzad et al. (2016) investigated the effects of royal jelly on frozen buffalo sperm and found that sperm motility, sperm viability, plasma membrane integrity, and acrosome integrity significantly increased at royal jelly concentrations of 0.05, 0.1, 0.2, and 0.3 compared to the control group (P < 0.05). This was attributed to the effects of proline, cysteine, and other antioxidant compounds in royal jelly, which reduced the negative effects of thawing on sperm quality (20). These findings are consistent with the results of the present study, which also showed the beneficial effects of royal jelly on human sperm due to its high antioxidant content.
In another study, Amirshahi et al. (2014) reported that male infertility can be influenced by diet and nutrients. In their study, 83 infertile men were given royal jelly at doses of 25, 50, and 100 milligrams, and no significant side effects were observed. After three months, there was an increase in progressive sperm motility, as well as in testosterone and hormone levels, although there was no significant increase in sperm count or follicle-stimulating hormone levels. Therefore, it can be concluded that royal jelly is a safe and effective option for the treatment of male infertility (21).
Amirshahi et al. investigated the effects of royal jelly on the sperm parameters of adult rats treated with lithium carbonate. Lithium carbonate was reported to impair the maturation and growth of sex cells by affecting the blood-testicular barrier, causing significant changes in total sperm count and decreasing sperm motility. However, in this research, royal jelly increased sperm count, viability, and motility, mitigating the toxic effects of lithium carbonate. As a result, the researchers proposed that royal jelly could be used in the treatment of infertility (21), which aligns with the findings of the present study.
Freezing and subsequent thawing may break down the antioxidants in sperm. In the second phase of this study, the addition of royal jelly reduced the negative effects of sperm freezing and thawing, and a significant difference was observed between TAC and antioxidant levels. In another study, Agarwal demonstrated that adding royal jelly to rabbit sperm increased the mean total antioxidant by up to 4% compared to the control group (22).
In summary, our findings indicated that royal jelly could increase the total antioxidants in semen, consistent with studies by Agarwal and Griveau (22, 23). Low levels of antioxidants (e.g., ascorbic acid) in seminal fluid could lead to hypersensitivity of sperm cells to oxidative damage, with reduced sperm motility as a possible outcome (24). The results of our study showed a significant difference in the MDA level, although no significant difference was observed between the two groups of thawed sperm. However, these groups were significantly different from the fresh sperm group. Furthermore, the increased addition of royal jelly decreased MDA, a factor that increases oxidative processes, though the reduction was not considered significant.
Based on the present study and previous findings, it is suggested that accurate measurement of ROS and TAC levels in seminal plasma (both enzymatic and non-enzymatic antioxidants) be considered in diagnosing infertile patients with high levels of TAC or ROS. Additionally, appropriate antioxidants could be used at specific doses to improve sperm fertilization in infertility clinics. Therefore, measuring antioxidants and assessing the oxidative status of seminal fluid in infertile men, including asthenospermia patients, could be beneficial for diagnosing, treating, and improving male fertility.
5.1. Conclusions
The treatment of infertility is associated with relatively low treatment costs and minimal side effects for the patient, and validated studies have confirmed the effectiveness of such treatments. According to the results of this study, adding a specific dose of compounds like royal jelly to semen could enhance sperm quality and motility through various antioxidants, especially after the freezing and thawing process. Royal jelly should be studied extensively to uncover its full range of intrinsic properties. Numerous researchers worldwide are currently investigating the compounds in royal jelly, and it appears that its discovered properties have positive effects on sperm function.