1. Context
In December 2019, a disease similar to acute fatal pneumonia was observed in Wuhan, China, which was recognized as a contagious viral disease transmitted by bats and soon spread like an epidemic throughout China and the world. It was initially referred to as acute respiratory syndrome, but after genetic sequencing and isolation of viral markers, the disease was officially named Covid-19 by the World Health Organization (WHO). And on January 30, 2020, WHO declared Covid-19 an international health emergency (1, 2).
Although the prevalence of this disease is very high among the elderly population, the involvement of children in this disease is very important [but the infection of children in this disease is of the utmost importance] (3, 4).
Most parents do not prevent their children from being in crowded situations due to the mental perception that Covid-19 affects older people and do not pay due attention to health protocols, which causes many children to become inflicted by this disease (5, 6).
It should be noted that Kawasaki vasculitis is a potential seasonal inflammatory disease that occurs in children under 5 years of age, first discovered in 1960 by Dr. Tomisaku Kawasaki in Japan (7). The disease causes an inflammatory reaction by activating macrophages, which may have remained in the tissues following previous infections (8).
Damage to the vascular system in the human body Due to the large extent of this system can pose irreparable dangers to other organs of the body; on the other hand, according to research on cardiovascular damage by Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (9, 10) and Kawasaki (11) vasculitis and also in the association of hypertension and SARS-CoV-2 we see an increase in comorbidity in patients (12-16).
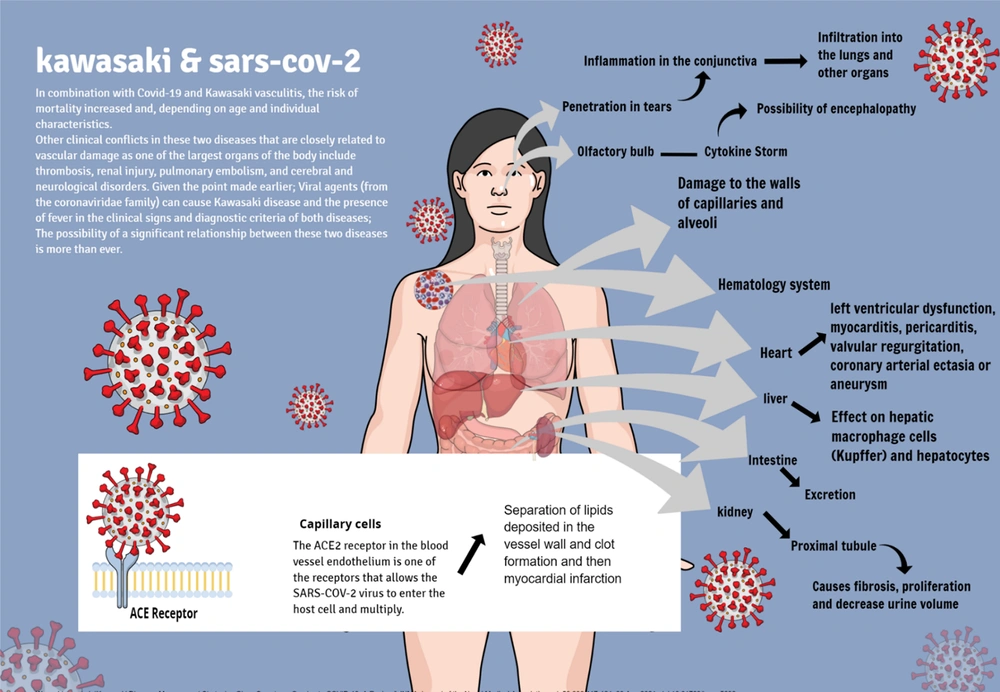
The association between Covid-19 and Kawasaki vasculitis increases the risk of mortality by 15 to 30 percent, and this increase in mortality is dependent on the patient's age and individual characteristics, although the exact mortality rate has not yet been proven (9-15). Other clinical conflicts between these two diseases that are closely related to vascular injury, which is one of the largest organs of the body, include thrombosis, renal injury, pulmonary embolism, and Cerebro-neurological disorders (16-28).
Given the point made earlier, viral agents (from the coronaviridae family) can cause Kawasaki disease, and [given] the presence of fever in the clinical signs and diagnostic criteria of both diseases, the possibility of a significant relationship between these two diseases is more than ever (29-32). The overlap of these two diseases is worrying because it may indicate the emergence of a new phenomenon in terms of rheumatology and immunology in the pediatric community, which indicates the need to study and compare these two diseases, and because of that, some researchers have described COVID-19 as a disease that causes "vascular inflammation" in children (5, 31-34). Of course, COVID-19 disease also has the potential to cause [various kinds of] abnormal inflammation[s], including skin rashes, which is also seen in Kawasaki vasculitis (35). In this article, we review the incidence of Kawasaki vasculitis in children with COVID-19 and some of the causes of these two diseases that researchers have found so far in the form of hypotheses.
2. Evidence Acquisition
2.1. Search Strategy
Search through various scientific databases such as Springer, Scopus, Wiley, science direct, PubMed, ProQuest, Cochrane Library, Embase, Clinical Key with keywords Covid-19, Kawasaki vasculitis, Mucocutaneous lymph node syndrome, pediatric, RNA viruses, cytokine storm, 2019-nCoV Diseases, SARS-CoV-2 Infection, SARS COV2 was performed. The search was limited to the English language. Search results were examined by a Virology Ph.D. and two general practitioners, and in cases of disagreement, decisions were made based on the opinion and consultation of an infectious disease physician. Inclusion criteria include thematic relevance and access to the full text of the article and the [time] period [of] 2019 to 2021. The titles of the articles, the abstracts, and the full text of the article were reviewed, and in the end, if unrealistic results were recorded, unavailable articles were removed. While reading the full text of the articles, 23 articles were manually entered into the search process and [were] used in the discussion section. At the end of the study, a search was conducted again using recently published articles.
3. Results
3.1. The Hypothesis of the Role of the Inflammatory System
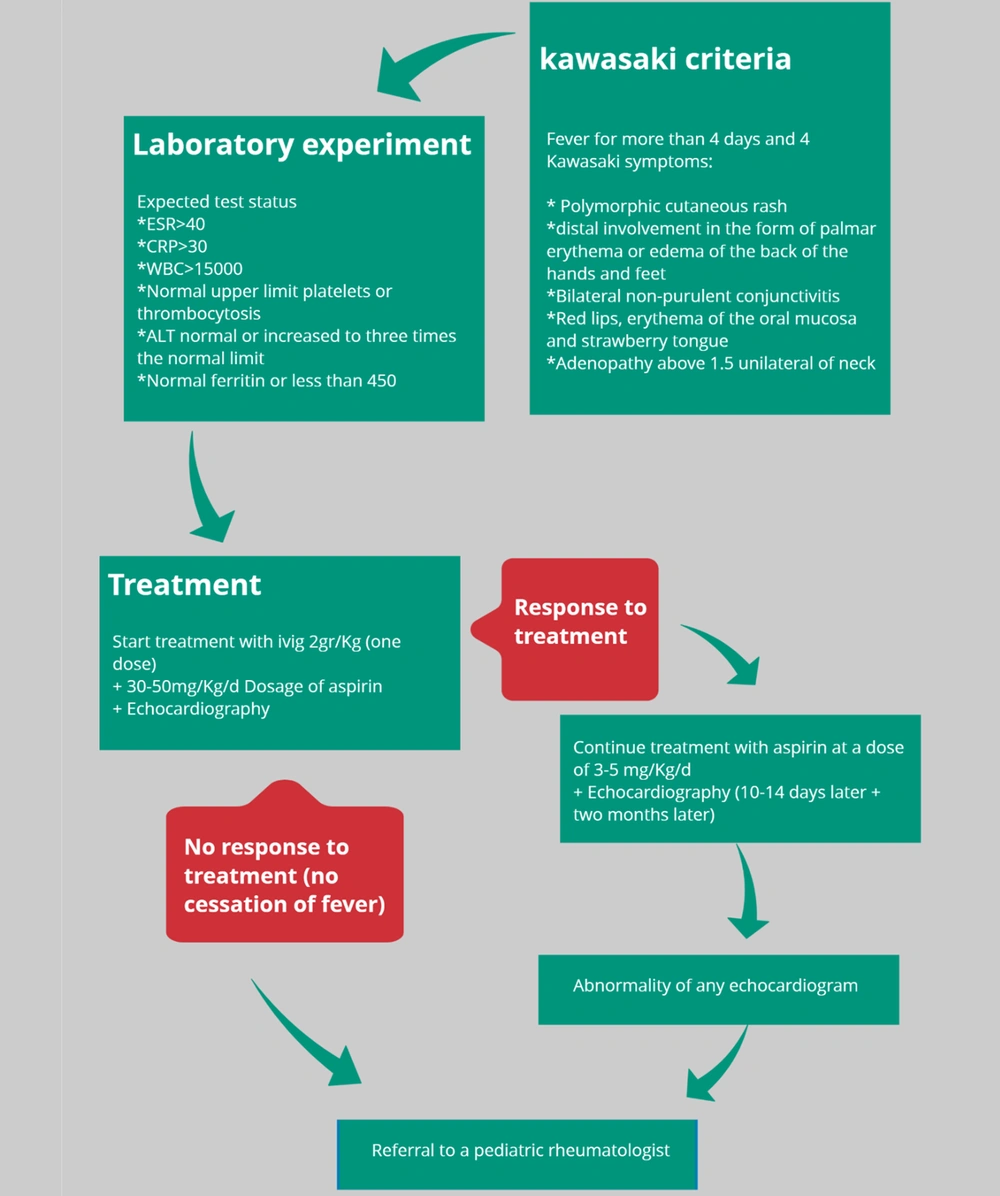
Previous studies have shown that SARS-COV-2 is an advanced and modified [mutated] generation of severe acute respiratory syndrome (SARS) and middle east respiratory syndrome (MERS) (36). Rollie and Schulman also hypothesized in 2018 that Kawasaki vasculitis may be caused by an unknown RNA virus (37). On the other hand, to test this hypothesis, in 2020, two studies were conducted on a community of children exposed to RNA viruses, and among them, symptoms similar to Kawasaki disease were observed. The value of this becomes clearer when laboratory findings also detect the presence of viruses from the coronavirus family. Another important point is that adults with severe COVID-19 had high levels of cytokines in their blood, a condition called a cytokine storm. Interestingly, the same condition has been seen in children with Kawasaki vasculitis (38-40). In 2020, during a study of 4 children with COVID-19, they were observed to develop cytokinin secretion syndrome, which worsened the patients' vital signs and the expression of Kawasaki vasculitis symptoms (41). In 2020, Ramcharan et al. (42) presented the aforementioned findings under a different heading: They selected 15 children with a mean age of 8.8. They examined the association between COVID-19 disease and Multisystem inflammatory syndrome in children (MIS-C). All 15 patients had a fever, 13 had gastrointestinal symptoms, and 8 had diagnostic criteria for Kawasaki vasculitis (Figure 1).
Among the 13 patients with gastrointestinal symptoms in the last two months, 2 patients had the SARS-COV-2 genome in their polymerase chain reaction (PCR) testing, and 3 patients had contact with people with positive symptoms, but in terms of serological tests (IgG, IgA, IgM), all 12 patients tested positive (42). The researchers of this study believe that although the average age of the patients studied was compared to the average age of Kawasaki disease patients, more attention must be paid when children become inflicted by COVID-19 (42). A similar study was conducted by Belot et al. in 2020 on 158 French children; 67% of the 109 children whom COVID-19 had inflicted were diagnosed with Kawasaki vasculitis and cardiovascular complications (43). In all studies on this hypothesis, except for the results of a study that did not state this issue conclusively, the need to pay attention to Kawasaki vasculitis in patients with the SARS-Cov2 virus has been emphasized.
Now the questions that arise are:
Why do the symptoms of these two diseases appear in one patient with this degree of similarity?
Is the only factor involved in this connection the high level of cytokinin in these people, or do other factors also play a role?
3.2. Hypothesis of ACE2
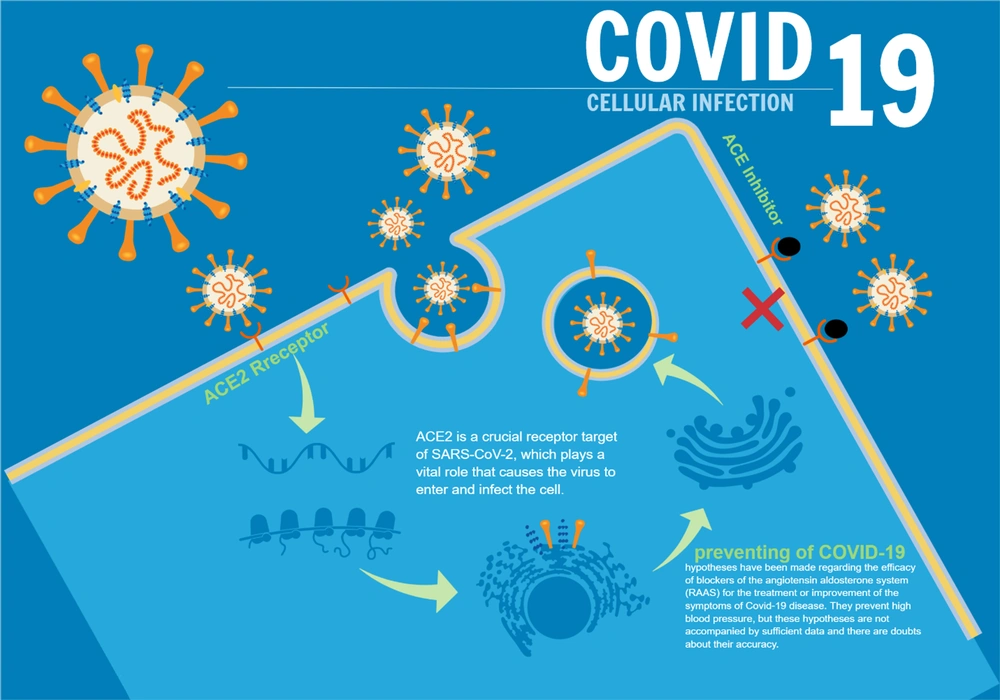
One of the enzymes active in regulating cardiovascular functions is angiotensin-converting enzyme 2 (ACE2). According to a 2020 study entitled “Angiotensin-converting enzyme 2 (ACE2) receptor for SARS-COV-2 virus: Its molecular mechanisms and therapeutic usability" by Zhang et al., it was determined that ACE2 receptors in the endothelium of blood vessels are among the receptors that allow the SARS-COV-2 virus to enter the host cell and multiply (44, 45). The SARS-COV-2 virus enters the vascular endothelial cell by binding to this protein receptor (ACE2) (44, 46, 47). Therefore, it can be concluded that wherever this ACE2 receptor is present, there is a possibility of SARS-COV-2 virus multiplication (48-52). Using the aforementioned information, we can better explain why the SARS-COV-2 virus first affects the lungs and then other organs (Figure 2). Because this virus, like other viruses in its family, has a high tendency to enter the upper respiratory tract, and the lungs, with a wide network of blood vessels, are a good environment for the virus to multiply, and consequently, clinical manifestations begin with respiratory symptoms. And then, it appears in other organs (53, 54). Since Kawasaki vasculitis is also a vascular endothelial disease that causes vascular complications by disrupting the regulatory mechanism of ACE2, it can be hypothesized that the causative agent of Kawasaki vasculitis may be the SARS-COV-2 virus (55). One of the suggested treatments for patients with COVID-19, which reduces the complications of the disease, is the injection of ACE2 into the patient. This causes the virus's surface glycoprotein receptors to bind to soluble ACE2 and becomes deactivated in practice because they lose the ability to bind to the body's normal cells. Although other methods have been suggested, this treatment strategy can be very promising (56); refer to Figure 3.
In the field of Covid-19 treatment, hypotheses have been proposed regarding the efficacy of angiotensin aldosterone system blockers (RAAS) in treating or improving the symptoms of Covid-19. These drugs block ACE2 and aldosterone receptors and prevent hypertension in patients with high blood pressure, although these hypotheses are not accompanied by sufficient data, and there is doubt about their accuracy (50, 57).
Another mechanism that disrupts the patient's vascular system is the loss of heparin, which is secreted from the inner surface of the vascular lumen by the vascular endothelial cells, which in turn prevents clots from forming. With the invasion of the COVID-19 virus into the vascular endothelium, the function of this molecule is impaired, which may be the cause of damage to end organs such as the kidneys (58-66).
3.3. The Hypothesis of the SARS-COV-2 Virus Role, Along with Other Risk Factors
In this hypothesis, the role of various factors in the development of Kawasaki vasculitis symptoms in patients with COVID-19 is mentioned, including genetic differences and cell surface markers of coronaviruses, which can provide a base for Kawasaki vasculitis in the future. The latter is probably formed by the weakening of the immune system by the COVID-19 virus.
Between 2001 and 2004, a study was performed on 53 patients with Kawasaki disease, 11 of whom had respiratory problems, and of those 11, 10 developed the classic symptoms of Kawasaki disease. Upon inspecting laboratory findings, in 8 patients, HCoV-NH viral markers were reported positive, and according to statistical studies, there was a significant relationship between the role of a viral pathogen of respiratory origin in Kawasaki vasculitis (67). Regarding pathogenesis, some studies suggest that the underlying cause of Kawasaki disease may be an unknown infection: Seasonal peak during winter and spring, the prevalence with the distinct geographical center, peak in 1 - 2 years old children, while infants less than 3 years are protected for months, possibly reflecting the protective role of transplacental antibodies. The disease is rare in adults as a possible consequence of previous exposure and acquired immunity. The specific infectious agent has not been identified as the cause of Kawasaki disease, and the viral origin of Kawasaki has not yet been established. It usually occurs in children and adolescents about 2 to 4 weeks after the onset of symptoms of COVID-19, not in the acute phase of COVID-19 (45). The probability that the viral pathogen is a member of the Coronavirus family has increased based on the evidence and medical interventions made since the beginning of 2020, some of which have been investigated in the present study;
In a 2020 case study, a 6-month-old girl with Kawasaki vasculitis was diagnosed with a viral infection. The patient was treated immediately for one day due to cough, fever, nasal congestion, and anorexia, but the treatment was useless. After urine analysis tests, nasal mucosa culture tests, and the negative presence of bacterial infections and influenza, the patient was under observation with a viral infection diagnosis. In addition to the rash on the fourth day, the appearance of sinus tachycardia and tachypnea with 100% oxygen saturation of the blood was another clinical finding of this child. These findings did not agree with the initial diagnosis of Kawasaki vasculitis, but they did not rule it out. After her fever rose to 38.8°C and the observation of weak turbidity on chest X-ray, both of which are clinical signs of COVID-19 disease (3, 68); For a laboratory examination of SARS-COV-2 infection, her blood sample was sent to the emergency department's laboratory. After her serological test was positive, the possibility of developing symptoms similar to Kawasaki disease from SARS-COV-2 was raised again. It seems that despite the complete inconsistency of clinical symptoms with the symptoms of Kawasaki vasculitis, there is a possibility that these two diseases (COVID-19 and Kawasaki vasculitis) are associated, or there is a cause-and-effect relationship between the two in children. According to the general conclusion of this study, physicians' attention to the different clinical symptoms of COVID-19 disease and its association with other diseases, such as Kawasaki vasculitis, seems necessary, and treatment policymakers are asked to pay special attention to this finding (3).
In another case study, the course of COVID-19 in a 5-year-old African-American boy was discussed, with the patient going to the hospital following a fever of 39.4°C for 8 days, swelling. (Palms, soles of the feet, and conjunctiva), loss of appetite, diarrhea, and abdominal pain. Clinically, he has the diagnostic criteria for Kawasaki vasculitis and is admitted with the same diagnosis. Laboratory data obtained from the patient's blood analysis showed anemia, leukocytosis, and increased infectious markers (ESR, CRP) and liver enzymes, with his flu test being negative and the observation of pericardial effusion in the patient's chest X-ray. The treatment staff was led to perform an reverse transcription polymerase chain reaction (RT-PCR) test on him, and after completing these procedures, he was diagnosed with COVID-19. The treatment plan was: Serum therapy with injectable immunoglobulin, diphenhydramine, and methylprednisolone. After 6 days, the symptoms of COVID-19 disappeared, and he was discharged from the hospital. This study's important point was the association between COVID-19 and Kawasaki vasculitis, which typically occurs in 5-year-old children. Two other reports were presented by Deza Leon et al., and other than coming to the same result as the previous studies, they also pointed to the increased risk of cardiovascular involvement in patients with these two diseases (COVID-19 and Kawasaki vasculitis) simultaneously (69, 70).
In 2020, Blandiaux et al. conducted a study to detect evident multiorgan involvement in the MRI of children with COVID-19. In this study, in addition to the symptoms of Kawasaki disease, the 4 children studied also had cardiovascular disorders. After serological testing, it was found that the 4 children had COVID-19 disease. The general conclusion is that: SARS-COV-2 disease alone may not cause cardiovascular complications but has effective antigens that can be the cause of extrapulmonary complications, especially cardiovascular complications (71).
Morand et al. conducted a descriptive-analytical study in 2020 entitled "COVID-19 and Kawasaki's disease" on 100 European patients between the ages of 6 months and 9 years, all of whom showed symptoms similar to Kawasaki's disease. Therefore, the study was started to discover the possible relationship between Kawasaki vasculitis and COVID-19. The researchers in this study believed that Kawasaki vasculitis occurs with a delay of 2 to 4 weeks in children between the ages of 6 months and 17 years, as well as genetically predisposed who have been exposed to viral agents. The prevalence of Kawasaki disease in Europe is third after Japan and North America, and the study results have revealed a positive relationship between Kawasaki vasculitis disease and the presence of an upper respiratory tract infection with positive 2 Hcov-OC43 and 3 Hcov-NL63 PCR test and, therefore more studies should be done to discover the pathological relationship between these two diseases to reduce the mortality rate caused by these two diseases (72). In another descriptive-analytical study, SARS-CoV-2 RT-PCR, SARS-CoV-2, and IgG ELISA tests were performed on 17 children with a mean age of 7.5 years to investigate the relationship between the prevalence of Kawasaki vasculitis in patients with COVID-19. This study was performed in France, and the statistical results obtained in this study indicate a significant relationship between the incidence of Kawasaki vasculitis and COVID-19 (4).
On the other hand, in a similar study, in a medical center in France, a previous association between Kawasaki vasculitis disease and viral diseases of the RNA type was investigated. In this study, due to the epidemic of COVID-19, in addition to increasing the prevalence of Kawasaki vasculitis in the pediatric population, we see a more severe inflammatory response than other previous viral groups in patients with Kawasaki vasculitis. These epidemiological findings in France indicate that the emergence of a new inflammatory syndrome has alerted the health authorities of this country (73, 74). To substantiate this claim, the findings of the study by Verdoni et al. (75), in an observational cohort study, expressed their findings as follows: In Bermago province in Italy, which is the center of widespread COVID-19, it is important to observe the widespread incidence of Kawasaki vasculitis. Therefore, all patients with Kawasaki vasculitis at that time should be divided according to the onset of COVID-19 and the onset of clinical symptoms of Kawasaki vasculitis. Category: Before (group 1) and after (group 2) epidemics of COVID-19 disease were divided, and for the presence of infection in the nasopharynx (upper respiratory system), serological tests SARS-CoV-2, IgM, and IgG were performed. Group 1 consisted of 12 girls and 7 boys with a mean age of 3 years, and Group 2 consisted of 7 boys and 3 girls with a mean age of 7.5 years. Maximum adaptation in terms of the geographical location of patients was done in two groups so that except for one patient, all patients were from Bermago province.
By designing this study method, they were able to obtain a significant relationship between these two diseases, so the comparison of the statistical results of the present study with the results of the last 5 years shows a 30-fold increase in the number of Kawasaki vasculitis patients after the COVID-19 disease epidemic and the coronaviridae family may have been involved in the pathogenesis of Kawasaki vasculitis in the last 20 years, although it should also be noted that: In 2014, a group of Japanese researchers upon discovering the link between the presence of two viruses in the coronaviridae family and the HCoV-NL63 and HCoV-229E genes in patients with Kawasaki disease, although there was no significant difference between patients and controls in terms of anti-HCoV-NL63 antibody positivity, anti-HCoV-229E antibody titers were significantly higher in Kawasaki patients than in controls (75). With the outbreak of COVID-19 in 2019, Chiotos et al. conducted a study on the clinical symptoms of 6 children aged 5 to 14 years in the United Kingdom. Statistical data show gastrointestinal symptoms in all patients and symptoms of Kawasaki vasculitis in 5 patients. The results of this study, in addition to proving the incidence of Kawasaki vasculitis in COVID-19 patients, indicate a favorable response to methylprednisolone and intravenous immunoglobulin injection, which could eliminate cardiovascular problems in patients, and 5 patients out of 6 patients in this study were discharged. Among all the mentioned studies, except for one case, all mentioned Kawasaki vasculitis due to COVID-19 (76). Evidence for a temporal relationship between the prevalence of Kawasaki-like disease and the COVID-19 epidemic, with a 13-fold increase in patients admitted with KD during the COVID-19 epidemic, has been presented (77). Children with KD-SARS-CoV-2, compared to children with classic KDs, have certain characteristics (77). Patients with KD-SARS-CoV-2 were of sub-Saharan African origins, were older, and often presented with gastrointestinal and neurological symptoms and myocarditis-compatible manifestations. Biochemical studies showed higher levels of inflammatory markers, recurrent lymphopenia, and elevated ferritin levels (77). Recently, several series of patients with [2] MIS-C have been reported, especially in countries with a high prevalence of COVID-19. All studies have not evaluated the extent to which these patients meet the KD criteria. Among these studies, patients who met the KD criteria accounted for 22 to 100% of MIS-C patients (77).
However, the above hypothesis, less than the previous two hypotheses, justifies the mechanism of association between COVID-19 and Kawasaki vasculitis.
4. Conclusions
According to recent findings in the aforementioned articles, following the widespread outbreak of COVID-19 in the world in 2019 and the already existing knowledge about the Coronaridae family and previous hypotheses about the relationship between the pathogen of this disease and Kawasaki disease, it can be concluded that there is a possible link between these two diseases. There are three hypotheses in this regard: The first is that the ACE2 receptor and the whole renin system angiotensin aldosterone (RAAS) is the SARS-cov2 virus's mechanism of entry and proliferation which can explain the pathogenesis of COVID-19 and Kawasaki vasculitis. Also, the relationship between the clinical symptoms and the behavior of the human immune system against Kawasaki vasculitis and COVID-19 can be explained by the fact that the SARS-COV-2 virus causes serum heparin to decrease by damaging the vascular endothelial tissue, which in turn causes intravascular thrombosis, and maybe the cause of Kawasaki vasculitis of the Coronavirus family. Another hypothesis is that SARS-COV-2 and other viruses of its family provide the environment for Kawasaki vasculitis with mechanisms that have not yet been identified. All the hypotheses are based on the information available to date, and our information in this regard is not enough; we need to design and conduct more studies and adopt larger statistical communities to confirm with certainty the existence of these relationships and this disease's pathophysiology. Over time, as reports of SARS-COV-2 infection in children increase, the things to learn about the virus also increase. Classic respiratory symptoms, less pronounced manifestations that have sometimes been reported due to pernio erythema, Chel Blaine, and other skin conditions such as the Covid finger (45). Determining the pathophysiology of Kawasaki disease by focusing on the pathogenicity of SARS-COV-2, in addition to limiting the domain of the pathogens causing this disease and trying to discover ways to treat it, can promise to preserve the life and the longevity of an important part of the Society namely the children in the future.