1. Background
Hemophilia is the most common and most serious inherited coagulation factor defect with an X-linked, recessive transmission, mainly involving the reduction or absence of coagulation factors VIII and IX, which are known as hemophilia A and B, respectively (1). Based on the level of factor 8 or 9, hemophilia is divided into three severe forms (less than 1%), moderate (1 - 5%), and mild (more than 5%). Without treatment, hemophilia patients, particularly in the more severe forms, are at risk of life-threatening bleeding (2, 3). In the last 50 years, the availability of clotting factor concentrates and home prophylaxis has caused a quick bleeding stop and increased life expectancy in these patients (4). In the past, the cause of death of hemophiliacs was hemorrhage or infection with human immunodeficiency virus (HIV) or hepatitis C; however, today, the cause of death has changed in most societies, and aging diseases, particularly cardiovascular diseases, are among the leading causes of mortality in hemophiliacs (5, 6). However, in a large study in the north of Iran, the most common cause of death of hemophilia patients was shown to be bleeding disorders and trauma. Only 2.5% of deaths were due to heart diseases, and the current study emphasizes that the causes of death of hemophilia patients are likely to be different in developed and developing countries (7).
In past studies, reports have been published regarding the protective effect of hemophilia in the occurrence of atherothrombotic heart disease. On the other hand, in some studies on hemophilia patients, ischemic heart disease is independently associated with age, hypertension, diabetes, and hyperlipidemia. In addition to diabetes and hypertension, high levels of factors 8 and 9 inhibitors, which may occur during factor concentrate infusions, and HIV infection may increase the risk of ischemic heart disease in this population (8, 9).
In addition, less research has been conducted on the susceptibility of hemophiliacs to congenital heart diseases with echocardiography imaging.
2. Objectives
Considering the prevalence of hemophilia patients in the country and region, in the present study, we evaluate congenital and acquired heart diseases (based on echocardiography-electrocardiography [ECG]) and major ischemic risk factors, including hypertension/hyperlipidemia-obesity in a hemophilia center in our region.
3. Methods
This research is an analytical epidemiological study in which the data are collected cross-sectionally. It is easy to take a non-random sample, and the sample size is equal to the statistical population. In this research, the files of 61 patients with factor 8 and 9 hemophilia were referred to the Dezful Hemophilia Center in 2020 - 2021 for periodical examinations or to take the coagulation factor. Of these, 50 patients were eligible for the study. Consent was obtained from all patients or their parents, depending on their age. Hemophilia patients with acute or other systemic problems were excluded from the study. For all studied patients, in addition to the necessary examinations, electrocardiogram and echocardiography were performed at the Echo Center of Dezful Hospital.
Liver, kidney, and thyroid function tests, lipid profile, and fasting blood sugar of hemophilia patients were extracted from the documents in the files. For the incomplete tests in the files, the necessary tests were requested again.
3.1. Review of Risk Factors
If the factor level is less than 1%, the severe form of hemophilia is considered, and if the factor level is 1 to 5%, the mild form is considered (10). Dyslipidemia, high fasting total cholesterol (> 200 mg/dL), triglycerides (> 150 mg/dL), low-density lipoprotein (LDL) (> 130 mg/dL), high-density lipoprotein (HDL) (> 40 mg/dL) or receiving cholesterol-lowering drugs were considered. Diabetes mellitus, fasting glucose (> 125 mg/dL), or receiving anti-hyperglycemic agents were considered (11). The study subjects’ overweight and obesity were considered based on body mass index (BMI), [weight (kg)/height (m)]., and BMI was calculated by dividing weight (kg) by the square of height (m²). Based on the definition of the Centers for Disease Control and Prevention (CDC), if BMI is 25 to 29.9 kg/m2, it is regarded as overweight, and BMI ≥ 30 kg/m2 is regarded as obesity (12). Patients' blood pressure was measured and recorded by a cardiologist with a mercury sphygmomanometer (Richter, Germany). After that, the participants sat and rested for at least 5 minutes; their systolic and diastolic blood pressures were measured twice using a standard sphygmomanometer. Then, the mean values of two measurements were used for all analyses. Hypertension systolic blood pressure higher than 140 mmHg, diastolic blood pressure higher than 90 mmHg (the high level was confirmed by repeating the test 30 minutes later), or receiving antihypertensive drugs were considered (13, 14).
3.2. Findings of Patients’ Echocardiography and Electrocardiography
In order to investigate cardiovascular disease, 50 hemophilia patients underwent echocardiography using the Vivid 3 echo device (USA) by an echo fellowship cardiologist. Patients' ECG was evaluated by a cardiologist based on universally accepted references. In echocardiography, the dimensions of cavities were measured by m-mode echocardiography. According to the American Society of Echocardiography guidelines, ejection fraction (EF) less than 50% is considered left ventricular systolic dysfunction, the tricuspid regurgitant gradient more than 30 mmHg is equivalent to pulmonary artery systolic pressure and more than 35 mmHg is equivalent to systolic pulmonary hypertension. Investigation of diastolic function based on pulse wave velocity (PW) of mitral valve flow and Doppler tissue of mitral valve annulus was evaluated and graded based on the criteria of the American Society of Echocardiography and the European Association of Cardiovascular Imaging (15).
For all patients, a standard 12-lead electrocardiogram from the body surface with a speed of 25 mm/s and a voltage of 10 mm/mV was performed in the supine position and analyzed by a cardiologist based on globally accepted reference values, and in case of any discrepancy with the standards, it was considered abnormal.
After the lab tests, echocardiography, and ECG preparation of the checklists, the obtained data were analyzed by SPSS statistical software version 21, and the Mann-Whitney and chi-square tests and the P-value less than 0.05 was considered.
4. Results
This study was conducted aiming to investigate the prevalence of cardiac diseases/risk factors in 50 hemophilia patients (Table 1). Between them, 43 patients (86%) had hemophilia A, 6 patients (12%) had hemophilia B, and 1 patient (2%) had acquired post-pregnancy hemophilia in a young woman. Also, 42 patients (84%) were found with severe hemophilia (factor deficiency below 1%), 5 patients (10%) with moderate hemophilia (factor level between 1% and 5%), and 3 patients (6%) with mild hemophilia (factor level greater than 5%). Furthermore, 5 hemophilia patients (10%) were female, and 45 patients (90%) were male (age range = 6 - 76 years, mean age = 28.12 ± 14.12 years). All patients were under control and received the necessary coagulation factor routinely. Based on the tests performed and the history of the patients, none of the hemophilia patients had kidney, liver, or thyroid problems or any other chronic disease as a confounding factor. None of them had a history of cigarette use, alcohol use, or any medication except the necessary coagulation factor.
| Characteristics | Criteria (N = 50) | P-Value |
|---|---|---|
| Gender | 0.935 | |
| Male | 45 (90) | |
| Female | 5 (10) | |
| Age | 28.12 ± 14.12 | 0.147 |
| Hemophilia A | 43 (86) | 0.05 |
| Hemophilia B | 6 (12) | 0.05 |
| Acquired hemophilia | 1 (2) | 0.05 |
| Mild hemophilia | 3 (6) | 0.05 |
| Moderate hemophilia | 5 (10) | 0.05 |
| Severe hemophilia | 42 (84) | 0.05 |
| The mean amount of receiving factors in a recent year | 8.87 ± 6.86 | - |
| Mean diastolic blood pressure (mmHg) | 74.54 | - |
| Mean systolic blood pressure (mmHg) | 109.24 | - |
| BMI (kg/m2) | 23.2 ± 4 | - |
| Type of treatment | On-demand | |
Demographic Information of Hemophilia Patients a
4.1. The Prevalence of Risk Factors
Of the studied patients, 6 (12%) had atherothrombotic heart risk factors of diabetes, hypertension, or dyslipidemia, 5 patients (10%) had hypertension, 5 (10%) had dyslipidemia, and 1 (2%) had diabetes mellitus with hemophilia. The age range of these 6 patients was 31 to 76 years, and the mean age was 46 years. They were mostly male with severe forms of hemophilia A and often more than one risk factor (Table 2).
| Noun | Age | Gender | Type of Hemophilia | Severity of Hemophilia | Number of Risk Factors | Hypertension | Diabetes | Dyslipidemia |
|---|---|---|---|---|---|---|---|---|
| 1 | 42 | Male | A | Severe | 1 | + | - | - |
| 2 | 76 | Male | A | Severe | 2 | + | - | + |
| 3 | 31 | Female | A | Severe | 2 | + | - | + |
| 4 | 45 | Male | A | Severe | 1 | - | - | + |
| 5 | 47 | Male | A | Severe | 2 | + | - | + |
| 6 | 37 | Male | B | Severe | 3 | + | + | + |
Investigation of Cardiovascular Risk Factors in 50 Hemophilia Patients a
4.2. Echocardiography and Electrocardiography Data in Hemophilia Patients
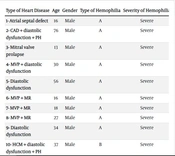
Based on the findings of echocardiogram and echocardiography, we considered heart disease in the study patients in two categories: Congenital and acquired. Acquired heart problems included EF below 50%, coronary artery disease (CAD), left ventricular diastolic dysfunction, systolic pulmonary hypertension, and congenital heart disease, including mitral valve prolapse (MVP), hypertrophic cardiomyopathy (HCM), atrial septal defect (ASD), and bicuspid aortic valve (BAV). According to the data shown in Table 3, a total of 11 patients (22%) were in the heart disease group (congenital or acquired). The number of cases of congenital heart disease was 8 (16%), and the number of acquired disease cases was 11 (some patients had several heart diseases at the same time).
4.3. Investigation of Congenital and Acquired Cardiovascular Diseases in the Studied Subjects
Mitral valve prolapse with or without mitral valve regurgitation (MVP+_MR) in 5 patients (10%), BAV in one patient, ASD in one patient, and a 37-year-old patient with hemophilia B with HCM were diagnosed based on echocardiography.
Grade I diastolic dysfunction was observed in 5 patients (10%), and systolic pulmonary artery hypertension was observed in 2 patients (4%). Only one of the patients had CAD. Based on angiography and echocardiography, this 76-year-old patient, who was the oldest hemophilia patient in the center, was being treated with anti-ischemic drugs due to 50% stenosis of the left anterior descending artery (LAD).
Moreover, this patient had two major risk factors, including hypertension and hyperlipidemia. No evidence of myocardial infarction or reduced ejection fraction was observed in the studied patients. Among the individuals with heart disease, 9 had hemophilia A. Also, all patients with heart disease were male and had severe hemophilia.
| Type of Heart Disease | Age | Gender | Type of Hemophilia | Severity of Hemophilia |
|---|---|---|---|---|
| 1- ASD | 16 | Male | A | Severe |
| 2- CAD + diastolic dysfunction + PH | 76 | Male | A | Severe |
| 3- Mitral valve prolapse | 13 | Male | A | Severe |
| 4- MVP + diastolic dysfunction | 30 | Male | A | Severe |
| 5- Diastolic dysfunction | 56 | Male | A | Severe |
| 6- MVP + MR | 16 | Male | A | Severe |
| 7- MVP + MR | 18 | Male | A | Severe |
| 8- MVP + MR | 27 | Male | A | Severe |
| 9- Diastolic dysfunction | 34 | Male | A | Severe |
| 10- HCM + diastolic dysfunction + PH | 37 | Male | B | Severe |
| 11- Bicuspid aortic valve | 39 | Male | B | Severe |
Information of Patients with Cardiac Disease Based on Echocardiography Findings of Hemophilia Patients
4.4. Electrocardiography Results
Except in two patients with HCM and ASD showing right and left ventricular hypertrophy in the ECG, respectively, the ECG was normal in the rest of the patients with hemophilia. Only in 4 patients under 18 years of age was T invert seen in leads V1 to V3, which is a normal finding in children (juvenile T-wave inversion).
5. Discussion
In this study, the prevalence of cardiovascular disease (congenital or acquired) and the risk factors of atherosclerosis, including diabetes, hypertension, hyperlipidemia, and obesity, were investigated in hemophilia patients in the region. A demographic study was conducted in the north of Khuzestan, Dezful, southwest Iran. Significant abnormality in echocardiography-ECG-risk factors was not observed in hemophilia patients compared to normal individuals in the community. According to the results of the present study, 84% of the patients had severe hemophilia, 86% had hemophilia A, and 90% of all patients were male. The age of hemophilia patients was mostly (80%) below 40, with a mean age of 28.12 ± 14.12 years, affecting the prevalence of risk factors and acquired heart diseases and cardiac ischemia. The most common risk factor was obesity (16%), followed by 10% hypertension and 10% hyperlipidemia, and the most common acquired congenital heart diseases were grade I diastolic dysfunction and MVP, respectively, each of which was observed in 10% of the hemophiliacs studied. In the investigation of risk factors in hemophilia patients, 6 people (12%) had atherothrombotic heart risk factors of diabetes, - hypertension, - or dyslipidemia, 5 patients (10%) had hypertension, 5 (10%) had dyslipidemia, and one (2%) had diabetes mellitus.
These 6 patients were all over 30-year-old males with a mean age of 46 years and often a severe form of hemophilia. In Amoozgar et al.’s study, of 50 hemophiliac patients with a mean age of 29 years in Shiraz, south of Iran, 14% had systemic hypertension; however, in our study, 5 patients (10%) had hypertension, and the mean age was 28 years. Our mean systolic and diastolic blood pressures were 109.24 and 74.54 mmHg, respectively, lower than the mean blood pressure of the patients in the above study (121.52 mmHg and 81.9 mmHg) (16).
The mechanism of increasing the risk of hypertension in hemophilia patients in some regions is unknown, but it seems that micro bleeding inside the kidney, particularly in severely poorly controlled hemophilia, causes renovascular hypertension, renal fibrosis, and systemic hypertension (17). Among other causes of the predisposition of these patients to high blood pressure may be their regular visits to the clinic and the finding of hypertension at the beginning (18-20).
In Sood et al.’s study on 200 hemophilia patients in the US aged 54 to 73 years, more than half of the patients had hypertension, more than half had dyslipidemia, only 19% had diabetes, and about 15% had thrombotic events (11). In the present study, about 10% had hypertension, 10% had dyslipidemia, only one patient (2%) had diabetes, and 1 patient (2%) had a history of atherosclerosis. However, in both studies, hypertension, and dyslipidemia were more common than diabetes, which, in addition to the difference in the sample size, the study location, and the genetics of the people, were clearly the most important reasons for the individuals’ age difference. In 2 studies, the subjects’ high age caused the prevalence of age-related risk factors.
Although the mortality of coronary disease seems to be lower in hemophilia patients than in normal individuals, several studies in the USA and Europe have shown that the prevalence of hypertension is higher in hemophilia than in age-matched patients. However, a recent study on 711 hemophilia patients in Japan has shown the prevalence of high blood pressure in hemophilia patients similar to other people in society (21). Moreover, it seems that the prevalence of hypertension in hemophiliac males is at least in the USA and Europe. In addition, the prevalence of hypertension in hemophiliac males seems to be at least in the USA and Europe; in severe forms of hemophilia, high age, high BMI, and the geographical area of study have been associated with hypertension in hemophilia patients, and in more severe forms, high age, high BMI, and the geographical area of study have been associated with hypertension in hemophiliacs (5, 21).
In a recent study on younger hemophiliacs between the ages of 0 and 21 years in Miami, cardiovascular risk factors in the population of hemophiliacs determined that diabetes was less common and blood pressure and obesity were more common in hemophiliacs than in non-hemophiliacs (22). In another study, hypertension, metabolic syndrome, and obesity were shown to be the most common cardiovascular risk factors in 48 hemophilia patients aged 6 to 40 years (23).
In a large study in the US, in older hemophilia patients with a mean age of 49.3 years, the prevalence of hypertension in hemophilia patients was 17% lower than in the general population; other risk factors of diabetes, atherosclerosis, hyperlipidemia, and obesity were also lower in hemophilia patients than in other community members (24). On the other hand, in another cohort study conducted by Shapiro et al. on 709 hemophilia patients over 30 years of age, the prevalence of hypertension was higher in age-matched controls (49% compared to 40%), and the prevalence of diabetes in hemophilia patients was similar to age-matched controls, which is consistent with the present study (5).
Although our study was in younger patients with hemophilia and the sample size was small, the prevalence of hypertension-diabetes and dyslipidemia risk factors was mainly over 30 years of age and severe hemophilia. The cause of the discrepancy in the prevalence of hypertension in hemophilia patients is different depending on the geographical area of the study, the genetics of the people, the difference in the definition of hypertension, the age of the patients, and the samples and methods of the studies. In our study, similar to other studies, diabetes was not common in hemophilia patients (only one out of 50 study patients) (14, 25).
Obesity in hemophilia patients is related to reduced joint movements and chronic pain caused by it (26). In Europe and the USA, the prevalence of obesity in hemophilia patients was 15% compared to 20% in the general population (27). The prevalence of obesity in hemophilia patients in our study was 16%; on the other hand, the prevalence of obesity in the general population in Iran was 20% (26). Our study was similar to some other studies; the prevalence of obesity was lower in hemophilia patients than in the general population (27, 28). Although the prevalence of obesity in patients in different countries is different based on the way of eating and lifestyle, in general, with aging and in recent years, obesity is increasing in both hemophiliac and non-hemophiliac patients. Of course, nutrition-inactivity-genetics and the regular treatment of these patients in preventing bleeding in the joints and immobility can be the reason for the difference in the prevalence of obesity in different areas of the study.
In Humphries et al.’s study, in the comparison of various cardiovascular risk factors in hemophilia patients compared to the control group, all risk factors were more common in the control group; however, in the case of diabetes and dyslipidemia, this difference was significant (29). Finally, in the present study, similar to other studies, cardiovascular risk factors in hemophilia patients appear similar to other people in society after the age of 30 - 40 years.
There was no study in the literature that included complete echocardiography to investigate congenital and acquired heart diseases in hemophilia patients. Echocardiography findings in 50 patients in our study, the most common congenital heart disease is MVP with or without mitral valve regurgitation), consisting of 5 patients (10%). Moreover, the most common acquired cardiac abnormality is diastolic dysfunction grade I, consisting of 5 patients (10%), and 2 patients (4%) had systolic pulmonary artery hypertension. The BAV, ASD, and hypertrophic cardiomyopathy HCM were observed in one patient each. In a study by Azami et al. (30) in Ilam, west of Iran, the prevalence of MVP in the general population in the age group of 20 - 30 years was about 12.5%, and in the age groups under 20 years and 30 - 40 years, it was 8.8% and 6.7%, respectively. The results of the mentioned study are consistent with the prevalence of MVP in our study in hemophilia patients (about 10%), and due to the proximity of the two regions, the prevalence of MVP in hemophilia patients is probably not significantly different from the general population.
The overall prevalence of congenital heart anomaly is estimated at 0.8% of all live births in the world (31). The possibility of bleeding in hemophiliac fetuses is involved in the development of fetal anomalies. However, in a previous study by Jedele et al., the prevalence of congenital heart anomalies in hemophilia patients was 0.75%, similar to the general population (32). Although the sample size of this study is small to investigate the prevalence of congenital heart diseases in hemophilia patients, it is higher than the overall prevalence in our study. The abnormality detection method and genetic and geographical differences may be the causes of this difference (33).
In Amoozgar et al.’s (16) study investigating echocardiography in hemophilia patients, diastolic dysfunction grade I and high-grade diastolic dysfunction were observed in 18% and 28% of hemophilia patients, respectively, but in our study, only 10% had grade I diastolic dysfunction. Of course, both studies are similar in the age of the patients; however, the reason for the difference may be the effects of anemia and confounding factors on the assessment of diastolic dysfunction and the difference in the echo cardiologist. The most important reason for the heterogeneity of different studies in reducing the prevalence of diastolic dysfunction compared to the past is the change in the diagnostic criteria of the American Society of Echocardiography guideline in 2016 compared to 2009 (34-36).
Grade I diastolic left ventricular dysfunction occurs when the left ventricle becomes stiff over time and has difficulty relaxing during diastole, and diastolic dysfunction definitely increases with age. In hemophilia patients, left ventricular diastolic dysfunction is expected with increasing age, which is consistent with the study results, and all patients with diastolic dysfunction were over 30 years of age (37).
ECG was normal except in two patients with HCM and ASD, showing hypertrophy of the left and right ventricles in the ECG, respectively. In the rest of the patients with hemophilia, the ECG was in the normal range. In Zong et al.’s (38) study in 2019, advanced ECG in hemophilia patients showed more help than standard ECG after the age of 40 in identifying cardiovascular diseases compared to controls (38). Regarding assessing CGP in Badescu et al.’s (39) study on 64 patients without cardiac symptoms of hemophilia over 30 years of age, changes in ECG were observed in 25 - 30%, and hypertension (56%) and dyslipidemia (72%) were the most common risk factors. Diabetes was observed in 14% of patients.
The difference between the results of their study and the present study may be the age of the studied patients, the geographical genetic differences, and the study method. In the study of atherosclerosis in patients, only a 76-year-old patient (2%) was the oldest person in the study with two risk factors of hypertension and hyperlipidemia, who had coronary stenosis with angiography. Despite the relative protection of ischemic heart disease in hemophilia patients, the prevalence of ischemic heart disease is increasing due to the increasing age of these patients. Although the exact prevalence of coronary syndromes in hemophilia patients is unclear, several studies have evaluated the prevalence of atherosclerosis in hemophilia patients as similar to the normal population. Mortality secondary to coronary syndromes is probably lower in the general population due to plaque stability and reduced thrombin production following plaque rupture (27).
In the present study, with a small sample size, the patients were mostly under 40 years of age, and considering the prevalence of atherosclerosis in mostly over 40 years of age, the decrease in the prevalence of ischemia may be involved in the study, but the fact that hemophilia accelerates atherosclerosis from a young age is probably not involved. In addition to the small sample size and younger patients, one of the weaknesses of this study was that it investigated risk factors and ischemic heart diseases. In addition, the effect of increased inflammation caused by receiving prophylactic coagulation factors or by hepatitis and HIV infections caused by receiving blood factors as a cause of increasing cardiovascular diseases was not investigated. The strength of the study was the complete echocardiography examination of hemophilia patients from a younger age and determining the prevalence of congenital or acquired heart diseases.
5.1. Conclusions
This study provides rare insights into the absence of a specific and prevalent cardiac disorder, hemophilia, in both echocardiography and ECG findings, as well as the absence of discernible risk factors amongst the examined patients. The findings of this atypical investigation indicate that the cardiac condition under scrutiny, which is both specific and prevalent, manifested no discernible indications of hemophilia through either echocardiography or ECG, nor were any patient profiles found to exhibit significant risk factors.
Considering the aging of hemophilia patients and the possibility of developing cardiovascular disease and risk factors with increasing age and the challenge of treating these patients due to the coagulation disorder and the rarity of the disease, wider multicenter studies are needed in this field. Undoubtedly, it is necessary to prepare practical guidelines for hemophilia patients in the prevention and treatment of cardiovascular disease in these patients.