1. Background
Epidermal growth factor receptor (EGFR) is a transmembrane tyrosine kinase receptor involved in cell survival and proliferation normally expressed by epithelial cells. Aberrant activation of this receptor, shown in epithelial cancers, can be induced by receptor overexpression, gene amplification, or activating mutations (1). Currently, some monoclonal antibodies targeting the extracellular domain of EGFR are approved for clinical use against various epithelial cancers (e.g., colorectal cancer (CRC) and head and neck cancer) (2).
Ginger (Zingiber officinale Roscoe) is a member of the Zingiberaceae family of plants.
Studies have shown that ginger is the most-used herbal drug in many countries.
Ginger's beneficial properties, including antioxidant, anti-inflammatory, and anticancer capacities, are supported by scientific evidence (3-6).
Furthermore, evidence shows that ginger has an antiemetic effect against chemotherapy side effects, such as nausea and vomiting in breast cancer patients (7-9). On the other hand, ginger has been suggested to synergize the anticancer effect of chemotherapy drugs for colorectal cancer (10).
2. Objectives
Since EGFR is one of the important markers in breast and colorectal cancer cells of epithelial origin, this study aims to investigate the effect of ginger extract on the expression level of this marker in MDA-mb231 and HT-29 cell lines.
3. Methods
3.1. Preparation Ginger Extract
Dried ginger rhizome (10 gr) was ground and subjected to extraction with absolute ethanol in the Soxhlet apparatus for 4 hr at 80 °C. Then, the ethanol in the extract was evaporated by a rotary evaporator at 90 °C. Finally, the obtained extract was dissolved in PBS.
3.2. MTT Assay
MDA-mb231 and HT-29 cell lines (Pasteur Institute Cell Bank, Iran) were cultured in RPMI-1640 supplemented with 10% FBS and 1% penicillin and streptomycin at 37 °C in 5% CO2.
The effect of different concentrations of ginger extract on MDA-mb231 and HT-29 cell lines was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The cell lines were treated with different (5, 10, and 20 µgr/mL) concentrations of ginger extract for 18 hours.
After that, 100 μL of MTT (0.5 mg/mL of culture media) was added to the cells and incubated for 4 hours. Then, 100 μL DMSO was added to each well, and OD was measured at 570 nm using a microplate reader (Awareness Technologies Stat Fax 2100, USA). The percent of cell viability was measured using the formula:
%Cell Viability = Absorbance of treated cells/Absorbance of control (without ginger extract) cells x 100.
3.3. Treatment MDA-mb231 and HT-29 Cell Lines with Ginger Extract
MDA-mb231 and HT-29 cell lines were treated with different concentrations of ginger (0, 5, 10, and 20 µgr/mL) extract in RPMI-1640 plus 10 % FCS for 18h. Then, the cells were harvested for EGFR expression analysis. This experiment was repeated three times for each cell line.
3.4. Assessment of mRNA Expression by Real-time PCR
The RNA was extracted from MDA-mb231 and HT-29 cell lines using TRIZOL reagent (Ambion, Life Technologies) according to the manufacturer's instructions. The concentration and quality were determined by spectrophotometry with a NanoDrop device (NanoDrop 2000c, Boston, New York, USA, Thermo Scientific). The mRNA expression level of EGFR in treated and untreated (control, 0 µgr/mL ginger extract) MDA-mb231 and HT-29 was detected using One-step Syber PrimeScript RT-PCR (Takara, Japan) according to the manufacturer's instructions. Briefly, RNA reverse transcription to cDNA using reverse transcriptase PrimeScript RTase. PCR amplification using Takara Ex Taq HS was done in one tube. PCR amplification products were monitored in real-time with Syber Green I on a light cycler 96 (Roche, Germany) qPCR (real-time PCR) thermal cycler. The target gene, EGFR primer: The forward GTGAGCAGATCGCAAAGG and the reverse CTTTGATCTTGACATGCTGC along with the housekeeping gene, β-actin, the forward primer 5'-GAGCATCCCCCAAAG-3' and the reverse primer 5'-GGGACTTCCTGTAACAACGCA-3' were used. The results for mRNA expression of the EGFR gene were analyzed by the comparative ΔCt method. The mean ΔCt for the EGFR gene was obtained by normalization of the threshold cycle (Ct) value to β-actin control. Lower ΔCt indicates more EGFR expression. In detail, the qPCR thermal cycling program was set as follows: an initial denaturation step at 95 °C for 15 minutes, a denaturation step at 95 °C for 25 seconds, an annealing step at 60 °C for 30 seconds, and an extension step at 72 °C for 30 seconds followed by a final extension step at 72 °C for 5 minutes.
3.5. Statistical Analysis
In this study, the significant differences between mean ΔCt of non-treated (0 µgr/mL) and treated MDA-mb231 and HT-29 cell lines with (5, 10, and 20 µgr/mL) ginger extract were analyzed with non-parametric Kruskal-Wallis test, and statistical significance was considered as P < 0.05. Statistical analyses were conducted using SPSS 19 (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. The Effect of Ginger Extract on the EGFR Gene Expression in Treated MDA-mb231 and HT-29 Cell Lines
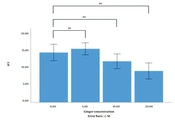
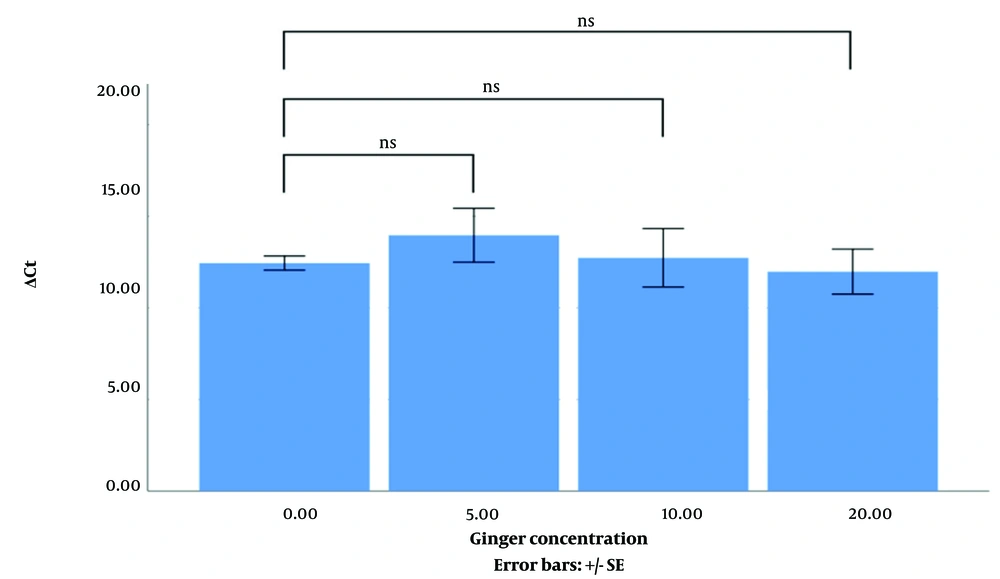
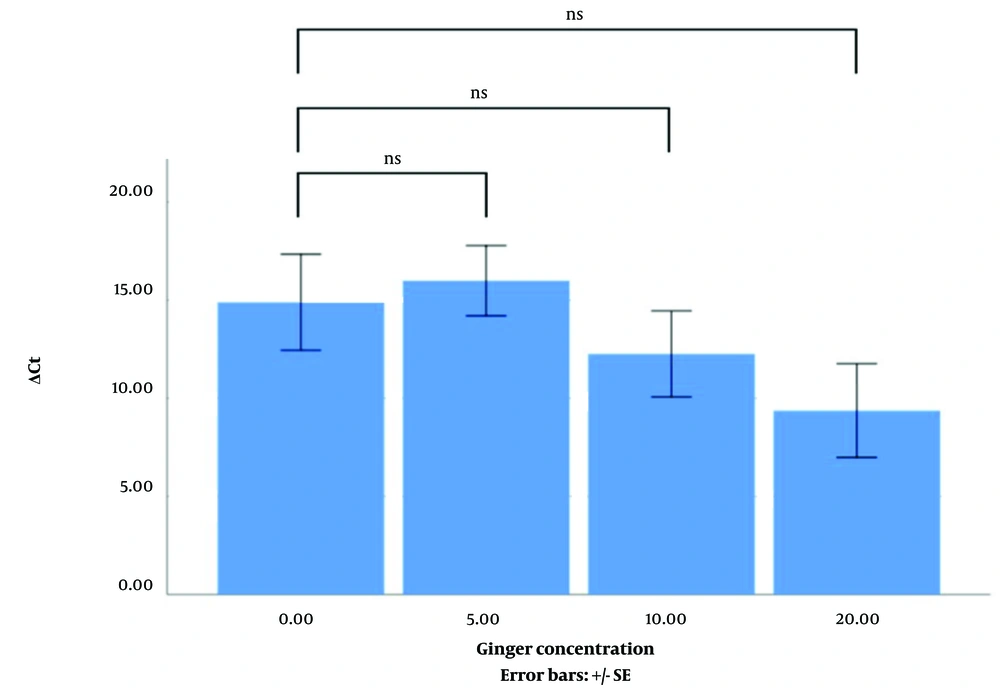
MTT assay showed that the percentage of MDA-mb231 and HT-29 cell lines viable after treatment with ginger extract (5, 10, and 20 µgr/mL) was not changed compared to the control (not shown). As Figures 1 and 2 indicate, the difference between the mean ΔCt of EGFR expression level of the MDA-mb231 and HT-29 cell lines treated with different concentrations of ginger extract (0, 5, 10, and 20 µgr/mL) is not significant.
5. Discussion
Cancer is one of the leading causes of death in the world. Breast cancer is the second leading cause of cancer-related mortality among women (11). Colorectal cancer (CRC) is a common cause of cancer death in both men and women, and its incidence is increasing worldwide (12).
Current treatment methods, including chemotherapy and radiotherapy, are widely used to treat breast cancer and CRC.
Although conventional treatments are essential for cancer patients, these methods have serious side effects that can be fatal during and after treatment (13).
It has been found that some natural plant compounds have anticancer properties against various types of cancer without significant side effects. Ginger, a famous spice broadly used in Asia, has been identified as having a lot of bioactive compounds, including phenolic compounds, terpenes, lipids, and carbohydrates. Among the hundreds of compounds found in ginger, four phenolic compounds are responsible for its biological effects: gingerol, shogaol, paradol, and zingerone. In vitro and in vivo studies have shown strong anti-inflammatory, antioxidant, and (6, 14-16) cancer prevention activity (e.g., improving the expression level of cancer risk markers) by these four important compounds (17, 18). Among cancer markers, EGFR is one of the most important receptors expressed in cancer cells of epithelial origin. Following phosphorylation, EGFR enhances tumor growth by activating proto-oncogenes. High levels of EGFR have been observed in different types of human cancer, which mediate the signal transmission into the cell cytoplasm and thereby promote tumorigenesis.
In this study, we wanted to investigate whether ginger affects the expression of EGFR in MDA-mb231 and HT-29, epithelial-origin cell lines. The cell lines were treated with ginger extract at three different concentrations. The experiments were repeated three times. The results showed that the chosen dose of ginger extract did not affect EGFR expression in MDA-mb231 and HT-29 cell lines.
Although some studies show that ginger extract components do not affect EGFR expression (13), since other studies show the anticancer effect of ginger extract components through the signaling caused by EGFR (18), it would be better that in this study the effect of ginger extract would also be investigated in a time-dependent manner.
Therefore, to conclude if the ginger extract applies its antitumor effect through EGFR expression change, it is suggested to repeat the experiments with higher concentrations of ginger in a time-dependent manner.