1. Background
Ulcerative colitis (UC) is an inflammatory bowel disease (IBD) that affects the intestines, especially the large intestine, including the colon and rectum. The etiology and pathogenesis of UC is mostly unknown (1). The disease begins with inflammation of the rectum and may reach the mucosa of the large intestine to varying degrees. Although the etiology of the disease is unclear, accumulating evidence suggests the involvement of several genetic and environmental factors, many of which have not yet been studied (2). It is hypothesized that UC is the result of the breakdown of tolerance to intestinal environmental antigens such as resident intestinal bacteria and changes in the barrier properties of the mucosal and epithelial layers. These changes allow luminal antigens to penetrate the intestinal mucosa and cause excessive production of pro-inflammatory cytokines and trafficking of leukocytes into the intestinal mucosa. This can eventually lead to out-of-controlled intestinal inflammation (3). Clinical signs of UC include bleeding and diarrhea, and in severe cases, systemic inflammatory reactions may also occur. In addition, more than 10% of UC patients develop non-intestinal symptoms, including joint involvement (enteropathic arthritis) and hepatobiliary problems, alongside cases of eye and skin lesions (4, 5).
Probiotics are increasingly being used to treat patients with UC. Probiotics are living nonpathogenic bacteria from which the intestinal and immune system can benefit. Some studies have shown that taking probiotics regularly and adequately at all ages is beneficial to health and prevents the persistence, replication, or pathogenicity of intestinal pathogens (6). Probiotics can improve local and systemic immunity, promote the secretion of anti-inflammatory factors, and increase intestinal mucosa barrier function. Probiotics can effectively maintain recovery in UC patients, indicating that optimization of intestinal microbiota is a key factor in the treatment of UC patients (7). Studies have also shown that different probiotics have different effects on the immune system. For instance, in our previous study, it was reported that Bifidobacterium lactis stimulated the secretion of anti-inflammatory cytokines more than Lactobacillus acidophilus. Still, it also induced the secretion of pro-inflammatory cytokines (8). In another study, it was reported that B. lactis stimulated the secretion of some anti-inflammatory cytokines but still increased the secretion of pro-inflammatory cytokines (9).
Molasses is a concentrated, dark, and viscous extract and a by-product in the process of preparing sugar from sugar beet or sugarcane. The quality of molasses depends on the ripening of the sugar beet, the amount of sugar extracted, and the extraction method. Despite the loss of most sucrose, molasses extract is rich in B vitamins and minerals, including iron, calcium, magnesium, potassium, manganese, and various other nutrients that can be needed due to the urgent need of patients with IBDs such as UC (10). Studies have shown that the use of molasses as a flavoring in food on the market not only increases sales but also reduces the harmful effects of artificial flavors (11).
It seems that IBDs can be caused by the insufficient suppression of immune responses by regulatory T cells (12). The forkhead box P3 (FOXP3) gene plays an important role in the development and function of regulatory T lymphocytes (CD4+ CD25+) (13). The FOXP3 gene provides instructions for producing the FOXP3 protein. The FOXP3 protein binds to specific regions of DNA and helps control the activity of genes that are involved in the regulation of the immune system (14).
There is extremely limited information on the effects of molasses on the immune system of patients with UC. In 2019, Shakurnia et al. investigated how the immune system of UC patients responds to molasses in vitro (11). Their results showed that sugarcane molasses added to B. lactis augments the secretion of TGF-β, the anti-inflammatory cytokine, by peripheral blood mononuclear cells (PBMCs) but does not decrease the level of TNF-α, the pro-inflammatory cytokine. Based on studies (15), the 30 - 40% sugar in the sugarcane molasses may be a trigger for inflammation.
2. Objectives
This study aimed to determine the effects of sugar-free molasses and B. lactis on the IFN-γ, TGF-β, and FOXP3 gene expression in the PBMCs of patients with UC.
3. Methods
3.1. Patients
Twelve patients with UC in the stable phase of the disease were enrolled in the Ganjavian Hospital, Dezful, Iran. These patients were diagnosed by a specialist based on colonoscopy and pathological indications. Of note, the patients were not in the acute phase of the disease and had not taken immunomodulatory drugs or corticosteroids for 4 weeks prior to colonoscopy. Moreover, this study was approved by the ethics committee of Dezful University of Medical Sciences, and all methods were performed in accordance with the relevant instructions and regulations.
3.2. Isolation of Peripheral Blood Mononuclear Cells
A volume of 10 mL of intravenous ethylenediaminetetraacetic acid (EDTA)--treated blood was collected from the patients. PBMCs were isolated using Ficoll (Baharafshan Co. Tehran) hypaque density gradient centrifugation. Whole blood was diluted with phosphate-buffered saline (PBS) solution (1:2 v/v). Then, the diluted blood was loaded on 3 mL of sterile Ficoll solution into the centrifuge tube, and then by centrifugation at 1000 g for 20 minutes, the PBMCs were separated. In the next step, the mononuclear cells were cultured in RPMI 1640 (10 mL), supplemented with 10% fetal bovine serum (Gibco, USA) and 1% penicillin-streptomycin (100 IU/mL) (Gibco, USA) at 37°C and 5% CO2.
3.3. Preparation of Bifidobacterium lactis
MRS broth (Merck, Darmstadt, Germany) culture media were prepared according to the manufacturer's instructions. Subsequently, it was divided into screw cap tubes and sterilized by autoclaving. The lyophilized B. lactis (DSM15954, Pishgaman Co. Tehran) was cultured in MRS broth under anaerobic conditions using the gas-pack method at 37°C for 48 hours. The tube containing the bacteria and medium was centrifuged at 3000 × g for 15 minutes. The supernatant was discarded, and the remaining pellet at the bottom of the tube was washed 3 times with PBS solution. Then, the bacterial concentration was determined by colony-forming unit (CFU) counts. Of note, in 1 mL of PBS solution in an optical density (OD) of 598 nm, when the absorption is 0.98, there are 1 × 109 CFU/mL bacteria, and thus the bacteria suspension was diluted by RPMI 1640 to reach the final concentration (16). Then, the tube containing the bacteria was exposed to ultraviolet (UV) light for 24 hours. The microbial suspension was then cultured on an MRS agar medium. After 48 hours under anaerobic conditions, no bacterial colonies were observed in the culture medium. This was an indication that UV had killed the bacterium. The microbial suspension was aliquoted in 2 mL microtubes and then stored in a freezer at -70°C.
3.4. Preparation of Sugar-Free Molasses
Sugarcane molasses was prepared from Caruncane Co. (Dezful, Iran). A volume of 10 mL of molasses was diluted with PBS solution (1:10, v/v), and then the sugar content of the molasses was depleted using the Steffen method (17). Of note, after the de-sugarization, the final sugar-free sugarcane (SFS) molasses had 0.1% sucrose. To standardize the method and its reproducibility, the dry weight of SFS molasses was determined. Three empty tubes were weighed separately by a sensitive digital scale. Next, 1 mL of SFS molasses was added to each tube. After 24 hours of incubating the tubes at 50°C, the SFS-molasses extract was completely dried, and then the three tubes were weighed again. Ultimately, the average dry weight of the molasses extract per mL was calculated. The final dry weight of SFS molasses was 300 μg/mL.
3.5. 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide Assay
The effect of different concentrations of SFS-molasses on mononuclear cells was evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The mononuclear cells were cultured in different concentrations of SFS molasses for 18 hours. The cells (2 × 105 cells/well) were also incubated in the absence of SFS molasses for 18 hours as a control. After that, 100 μL of MTT solution (0.5 mg/mL of culture media) was added, and the cells were incubated for 4 hours. Finally, 100 μL DMSO was added to each well, and OD was measured at 570 nm using a microplate reader (Awareness Technologies Stat Fax 2100, USA). The cell viability percentage was then measured using the formula:
% Cell viability = Absorbance of treated cells/Absorbance of control cells × 100
3.6. Co-culture of Mononuclear Cells with SFS-Molasses and Bifidobacterium lactis
The UC patients’ PBMCs (4 × 105 cells per well) were co-cultured with cell: Bacteria ratio 1: 50 and 300 μg/mL SFS-molasses in the final volume of 1.6 mL CM10 medium for 18 hours. Then, the six-well microplates were centrifuged at 800 × g for 5 minutes. Next, the supernatant of the co-cultures was aspirated and stored at -70°C until the cytokines were measured using the enzyme-linked immunosorbent assay (ELISA) method.
3.7. Measurement by ELISA
The tests were performed as indicated before and according to the instructions of the diagnostic kits. Zellbio ELISA kits (ZellBio GmbH, Germany) were used to measure IFN-γ and TGF-β (18).
3.8. Assessment of mRNA Expression by Real-time PCR
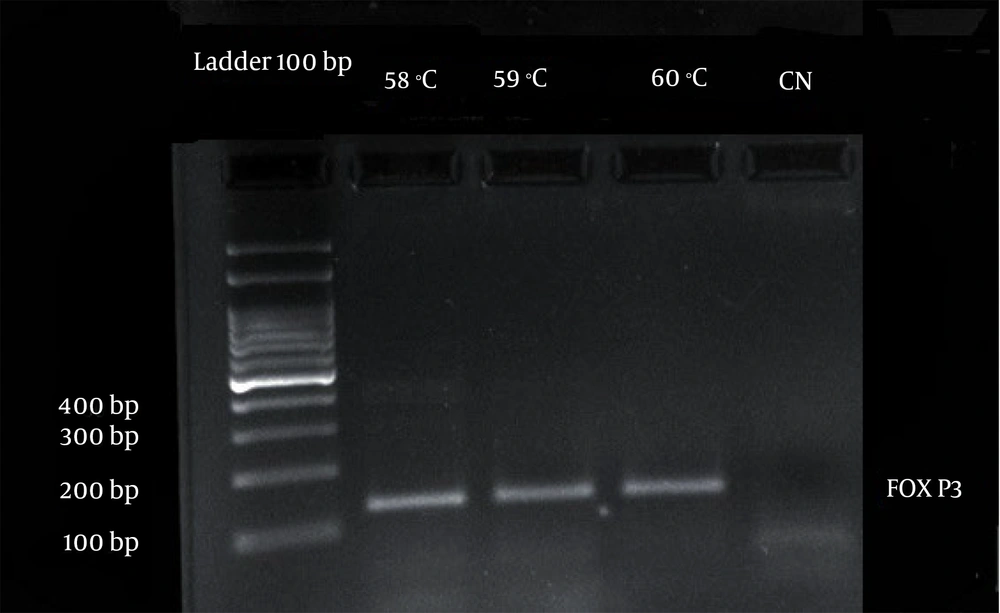
The RNA was extracted from PBMCs using TRIZOL reagent (ambion, life technologies) according to the manufacturer’s instructions. The concentration and quality were determined by spectrophotometry using a NanoDrop device (NanoDrop 2000c, Boston, New York, USA, thermo scientific). The mRNA expression level of target genes of treated and untreated (control) PBMCs was detected using one-step Syber PrimeScript RT-PCR (real-time PCR) (Takara, Japan) according to the manufacturer’s instructions. Briefly, RNA reverse transcription to cDNA using reverse transcriptase Prime script RTase and PCR amplification using Takara Ex Taq HS was done in one tube. Polymerase chain reaction amplification products were monitored in real-time with Syber Green I on light cycler 96 (Roche, Germany) qPCR thermal cycler. The target gene, FOXP3 primer: The forward TCTTCCTTGAACCCCATGCC and the reverse AAATGTGGCCTGTCCTGGAG TC along with the housekeeping gene, β-actin, the forward primer 5'-GAGCATCCCCCAAAG-3' and the reverse primer 5'-GGGACTTCCTGTAACAACGCA-3' were used. The results for mRNA expression of the FoxP3 gene were analyzed by performing the comparative ΔCt method. The mean ΔCt for the FoxP3 gene was obtained by normalization of the threshold cycle (Ct) value to β-actin control. Smaller ΔCt values indicate more expression of FoxP3. In detail, the qPCR thermal cycling program was set as follows: An initial denaturation step at 95°C for 15 minutes, a denaturation step at 95°C for 25 seconds, an annealing step at 58, 59, and 60°C (the 60°C was chosen) for 30 seconds, and an extension step at 72°C for 30 seconds followed by a final extension step at 72°C for 5 minutes. Finally, the quality of the DNA fragments was assessed on 1.5% agarose gel.
3.9. Gel Electrophoresis of the qPCR Products
To evaluate the quality of the PCR products, they were electrophoresed on 1.5% agarose gel (Figure 1).
3.10. Statistical Analysis
In this study, the significant differences between IFN-γ and TGF-β of non-treated and treated PBMCs with SFS-molasses were analyzed with Student’s t-test and ANOVA, and statistical significance was considered as P < 0.05. Statistical analyses were carried out using SPSS 19 (SPSS Inc., Chicago, IL, USA).
4. Results
4.1. The Effect of Sugar-Free Sugarcane-Molasses and Bifidobacterium lactis + SFS-Molasses on the Secretion of Interferon Gamma and Transforming Growth Factor Beta by Peripheral Blood Mononuclear Cells
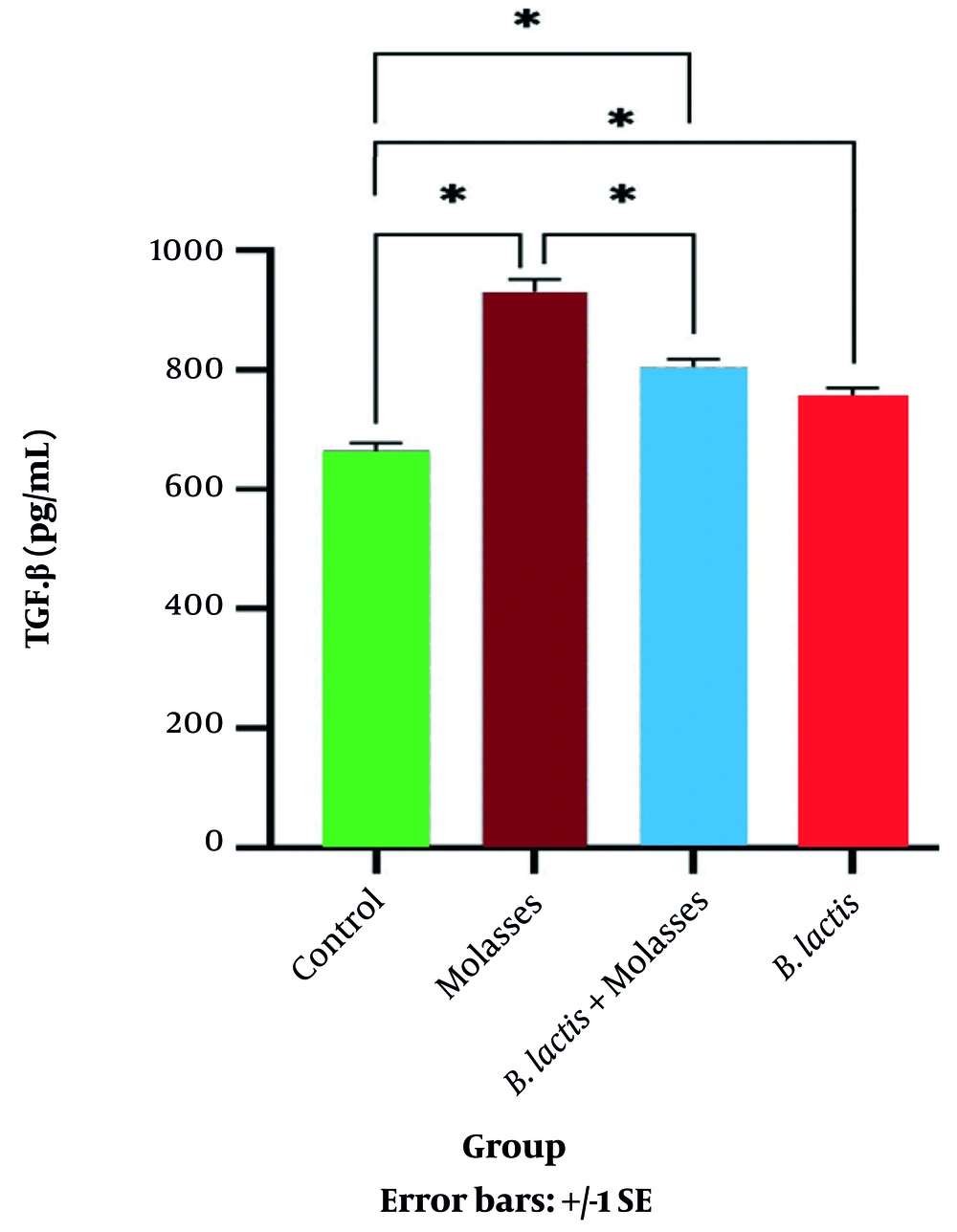
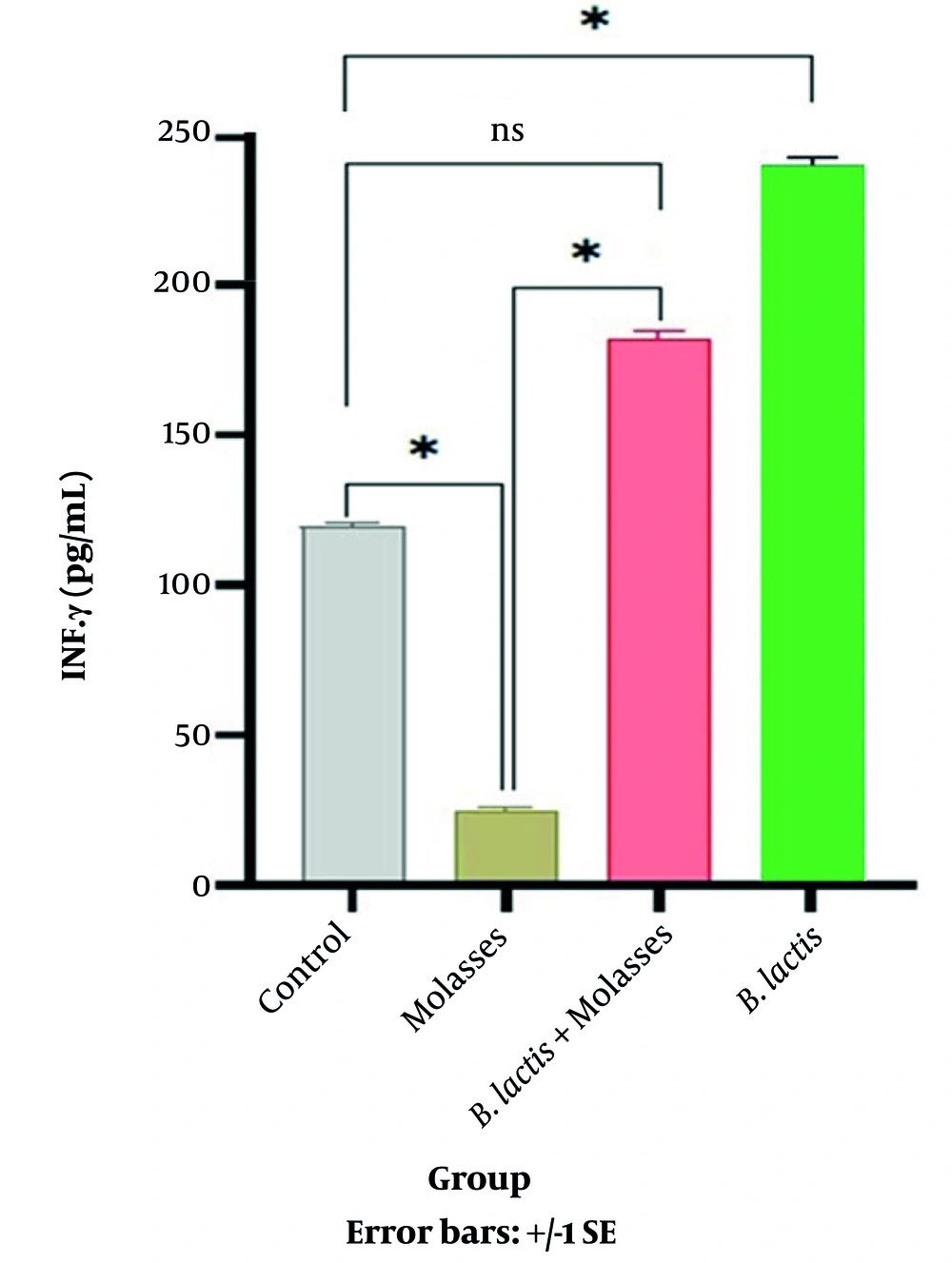
MTT assay showed that the percentage of viable PBMCs after co-culture with SFS-molasses (13.5 μg/mL) was not changed compared to the control group (not shown). Therefore, it was concluded that SFS molasses did not have any toxic effects on the PBMCs. As shown in Table 1 and Figure 2, the secretion of the anti-inflammatory cytokine TGF-β in the SFS-molasses group was significantly increased compared to the control group (P = 0.032). The secretion of TGF-β in SFS-molasses + bacteria and the bacteria alone groups increased compared to the control group (P = 0.039 and P = 0.049, respectively).
| Bacteria: Cell Ratio | SFS-Molasses μg/mL | TGF-β (pg/mL) | IFN-γ (pg/mL) | |
|---|---|---|---|---|
| SFS-molasses | - | 300 μg/mL | 932.85 ± 386.70 | 23.72 ± 8.09 |
| SFS-molasses+ B. lactis | 50:1 | 300 μg/mL | 815.15 ± 373.13 | 181.36 ± 61.41 |
| B. lactis | 50:1 | - | 751.12 ± 95.10 | 245.01 ± 50.01 |
| Control | - | - | 690.37 ± 306.74 | 119.05 ± 13.49 |
Abbreviations: SD, standard deviation; B. lactis, Bifidobacterium lactis.
a Data are presented as mean ± SD.
In addition, the secretion of IFN-γ in the SFS-molasses group was significantly reduced compared to the controls (P = 0.004). IFN-γ secretion increased in the SFS-molasses + B. lactis group compared to the control group, but it was not significant (Figure 3).
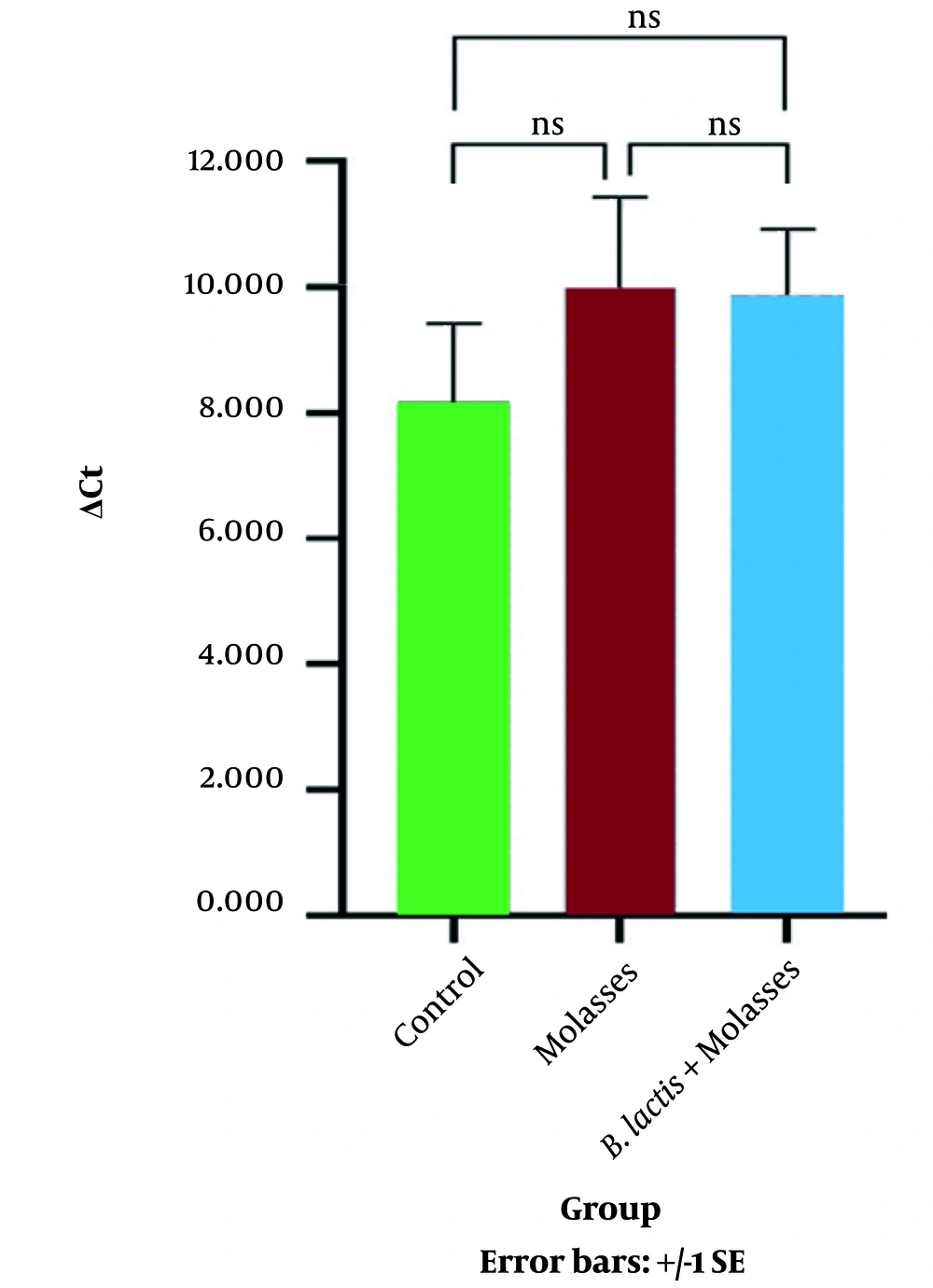
4.2. The Effect of Sugar-Free Sugarcane-Molasses and Bifidobacterium lactis + SFS-Molasses on the Expression of the FOXP3 Gene
The results represented in Table 2 and Figure 4 show that there was no significant relationship between the expression of the FOXP3 gene in the SFS-molasses group compared to the control group (P = 0.269). Also, the results indicated that there was no significant relationship between the expression of the FOXP3 gene in the SFS-molasses + B. lactis group compared to the control group (P = 0.302).
| Variables | Group | ||
|---|---|---|---|
| Control | SFS-Molasses | SFS-Molasses + Bifidobacterium lactis | |
| Ct (β-Actin) | 22.003 ± 2.831 | 20.241 ± 5.106 | 22.769 ± 6.254 |
| Ct (FOXP3) | 30.153 ± 4.454 | 30.346 ± 5.078 | 32.739 ± 4.538 |
| ΔCt | 8.150 ± 2.8600 | 10.105 ± 4.951 | 9.970 ± 4.047 |
Abbreviation: Ct, threshold cycle.
a Data are presented as mean ± SD.
5. Discussion
Ulcerative colitis is a recurrent and inflammatory disorder of the gastrointestinal tract. Between 20% and 85% prevalence of malnutrition has been reported in patients with UC (19).
Although the immunopathogenesis of UC remains unclear, current evidence indicates that altered T cell response to intestinal environmental antigens disrupts the balance of the mucosal immune system and causes inflammation in the intestine, which leads to chronic diarrhea and, finally, malnutrition. Nutrient (e.g., vitamin and mineral) deficiencies in patients with UC occur via several mechanisms (e.g., insufficient intake, impaired absorption via inflamed or functionally impaired epithelia). They may complicate and prolong the course of the disease (20, 21).
Sugarcane molasses, the final effluent of sugar refinement, is a concentrated and viscous substance full of vitamins, minerals, and antioxidants that could be a good supplement for nutritional deficits. Still, its effect on the immune system of UC patients is almost unknown (22-24).
The Shakurnia et al. study showed that sugarcane molasses augments both the anti-inflammatory and pro-inflammatory cytokines by PBMCs of UC patients (11). Other evidence indicates that the inflammatory responses of mononuclear cells to sugarcane molasses may be due to the high sugar concentration in molasses (11, 15, 25). The Shakurnia et al. study showed that sugarcane molasses caused a significant increase in TGF-β compared to the control group. On the other hand, not only did the combination of molasses and B. lactis increase TGF-β levels, but it also increased the secretion of the proinflammatory cytokine TNF-α. The amount of sugar in sugarcane molasses can vary between 30% to 40%, depending on its type. The main sugar is sucrose, but a small amount of glucose and fructose can also be present. Therefore, in the present study, we isolated the sugar in vitro using the Steffen method to reach 0.1% sugar in de-sugarized SFS-molasses. For the first time, its effect on the immune response of the PBMCs in patients with UC was investigated.
This study showed that SFS-molasses alone and the combination of SFS-molasses + B. lactis significantly increased the secretion of TGF-β in comparison with the control group. However, in the SFS-molasses group, the increase in the secretion of TGF-β was greater than that of the combination of SFS-molasses + B. lactis. In addition, SFS-molasses reduced IFN-γ secretion by PBMCs in comparison with the control group.
Transforming growth factor beta is a multifunctional cytokine whose main function is to regulate inflammatory processes, especially in the intestine. Therefore, up-regulation of TGF-β secretion by PBMCs after treatment with SFS molasses could be directly related to the anti-inflammatory effects of SFS molasses (26).
In the present study, the combination of B. lactis and SFS-molasses significantly increased TGF-β levels.
Regarding the pro-inflammatory effects, although B. lactis caused a significant increase in the level of IFN-γ, the combination of B. lactis and SFS molasses did not increase the secretion of IFN-γ significantly. Interferon gamma is one of the important cytokines whose secretion is closely related to the balance of T lymphocyte response (27). Our previous study (8) that compared the stimulatory effects of L. acidophilus and B. lactis on the PBMCs of patients with UC demonstrated that B. lactis had a better regulatory effect (although it had both pro-inflammatory and anti-inflammatory properties). The current study demonstrated that the pro-inflammatory effects of B. lactis were modified by adding SFS molasses (Figure 3).
Although this study confirmed the effects of SFS molasses on modulating immune responses, the FOXP3 gene was expected to be upregulated. Still, its expression did not show any increase compared to the control group. This can be due to several reasons. First, it is possible that the small number of patients enrolled in the study was insufficient to make a statistically significant difference. Moreover, factors other than FOXP3 may also be involved in regulatory T-cell activity. It is also possible that the method of separating sugar from molasses (Stephen's method) in the laboratory interfered with the expression of the FOXP3 gene. Unfortunately, we could not measure the effect of B. lactis alone on the expression of the FOXP3 gene in this study.
In the present study, due to the small sample size and the insufficient number of mononuclear cells isolated from the patient’s blood, it was not possible to investigate the dose- and time-dependent effect of SFS-molasses, the SFS-molasses + B. lactis, and the bacteria alone on the TGF-β and IFN-γ levels. Therefore, future studies with larger blood sample volumes are warranted to investigate this.
Despite the mentioned limitations, this study showed that SFS molasses increases the anti-inflammatory cytokine TGF-β and decreases the pro-inflammatory cytokine IFN-γ. When SFS-molasses and B. lactis were added together to the mononuclear cells, the SFS-molasses reduced the inflammatory effect of the bacteria. Therefore, based on these results, further investigation is warranted to determine if molasses can be a beneficial nutrient for ulcerative colitis patients.