1. Background
Septic arthritis (SA) or infectious arthritis (IA) constitutes a rheumatologic emergency due to rapid joint destruction, leading to significant morbidity and mortality (1). Essentially, this condition involves an infection of the synovial space, affecting the synovial membrane, joint space, and associated structures (2). Hematogenous seeding of the vascular synovial membrane due to bacteremia is the primary mechanism of progression for most septic joints (3). While individuals of all ages can be affected, SA predominantly occurs in older adults and very young children (4). The incidence of this condition peaks between the ages of 2 and 3 years and is twice as high in males compared to females (5, 6).
Any abnormal conditions such as joint injury or trauma, hemophilia, osteoarthritis, or concurrent diseases like cirrhosis, cancer, and uremia, increase the risk of contracting this infection (7). The exact prevalence of this disease is unknown, but some reports indicate between 5.5 and 12 cases per hundred thousand individuals. In over 60% of cases, only one joint is affected, with symptoms including heat, redness, and severe pain in the infected joint. Common accompanying symptoms are fever and peripheral blood leukocytosis (8). Septic arthritis should be considered in adults presenting with acute monoarticular arthritis affecting a single joint, typically characterized by redness, swelling, and pain. Delay or errors in initiating appropriate antibiotic therapy within the first 24 to 48 hours may lead to subchondral bone loss and permanent joint dysfunction (5, 9).
Among the bacterial agents causing SA, Staphylococcus aureus is the most common in adults, with Streptococci spp. being the second most common cause. Gram-negative bacilli account for 5 - 20% of cases. Other causative agents include anaerobes, Salmonella spp., Neisseria meningitidis, Brucella, and Mycoplasma hominis (10, 11). In countries without routine vaccination, Haemophilus influenzae type B is responsible for more than half of the cases. Group A Streptococcus and pneumococcus contribute to 10 to 20% of cases (12, 13).
Septic arthritis typically presents with fever (often mild, with only 30 - 40% of individuals having a temperature exceeding 39°C), erythema, swelling, and tenderness. Decreased range of motion of the affected joint(s) accompanied by reluctance to walk may also be observed. The severity of swelling and joint tenderness can vary. In most cases, the knee is the most commonly affected joint in both children and adults, followed by the hip, shoulder, wrist, and ankle. Bacteremia occurs most frequently in immunosuppressed individuals and hospitalized patients, particularly those undergoing invasive procedures, using intravascular devices, or having urinary catheters. Infection occurs when the patient is immunosuppressed or the joint is compromised (3, 4). Arthrocentesis and joint fluid analysis are the preferred diagnostic tests for early detection of SA (14). Any suspicious joint or bone should be aspirated for testing. Several studies have reported positive synovial fluid cultures in 42% to 52% of these patients (15, 16). The emergence of bacterial strains, particularly those that are highly virulent and resistant to antibiotics, has made treating these conditions more challenging than in the past, necessitating the availability of new antibiotic molecules to address these emergencies (17).
Given the importance of early diagnosis and treatment of SA and the significant increase in antibiotic resistance among hospitalized cases, identifying the causative pathogens of this disease and their antibiotic resistance patterns is crucial.
2. Objectives
This research was designed to investigate the prevalence of bacterial agents causing SA and their trends in antibiotic resistance.
3. Methods
3.1. Data Collection and Analyses
The records of patients admitted between 2018 and 2020 were extracted from the hospital archives and meticulously reviewed for cases of SA. Demographic information, including age, gender, hospitalization department, and Antimicrobial Susceptibility Test (AST) results, as well as details regarding the type of bacteria causing the infection, were extracted and recorded in the appropriate forms. Subsequently, statistical analysis was conducted using SPSS version 22 software. Descriptive statistics were employed to generate abundance charts. Additionally, the chi-Square (χ²) test was utilized to explore the relationship between bacterial contamination and qualitative variables. The methodology relied on data collection from patients' files, and the information regarding the microorganisms responsible for SA was derived from the results of bacterial and fungal cultures documented in the patients' files. This study specifically investigated bacterial organisms implicated in SA.
4. Results
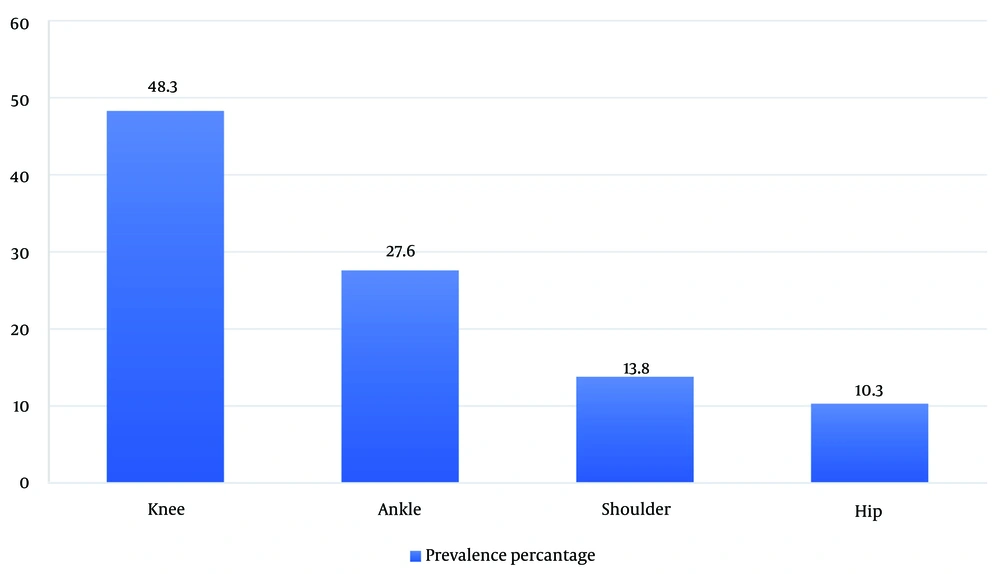
During this study, 249 samples were examined, revealing 29 positive cases of bacterial culture (11.6%) and one candida infection (0.4%). Among the 29 positive culture cases, men were affected 2.6 times more than women. The knee was identified as the most affected joint, followed by the ankle (Figure 1). Analysis of joint fluid culture indicated that S. epidermidis was the most frequent cause of infection, followed by S. aureus.
The prevalence of bacterial agents causing infection is presented in Table 1. Examination of the prevalence data across different age groups revealed Citrobacter fereundi isolation in the age group of 43 to 51 years, E. coli in age groups less than 18 years, 43 to 51 years, and 52 to 79 years, Enterococcus spp. predominantly in the age group of 52 to 79 years, Klebsiella spp. in the age group of 52 to 79 years, Proteus spp. in the age group of 34 to 42 years, S. aureus in age groups less than 18 years, 18 to 33 years, 34 to 42 years, predominantly in the age group of 52 to 79 years, and in the age group of over 80 years, and S. epidermidis in age groups less than 18 years, 18 to 33 years, predominantly in 34 to 42 years, 43 to 51 years, and 52 to 79 years. Most patients in this study belonged to the age groups of 52 to 79 years, followed by 34 to 42 years (Tables 2 and 3).
| Bacterial Agent | Prevalence (%) |
|---|---|
| Staphylococcus epidermidis | 12 (41.4) |
| Staphylococcus aureus | 7 (24.1) |
| E. coli | 3 (10.3) |
| Enterococcus spp. | 3 (10.3) |
| Klebsiella spp. | 2 (6.9) |
| Proteus spp. | 1 (3.49) |
| Citrobacterfereundi | 1 (3.4) |
| Total | 29 |
| Age (y) | Prevalence (%) |
|---|---|
| < 18 | 4 (13.9) |
| 18 - 33 | 3 (10.3) |
| 34 -42 | 6 (20.7) |
| 43 - 51 | 3 (10.3) |
| 52 - 79 | 12 (41.4) |
| > 80 | 1 (3.4) |
| Total | 29 (100) |
| Bacterial Isolates | < 18 | 18 - 33 | 34 - 42 | 43 - 51 | 52 - 79 | > 80 |
|---|---|---|---|---|---|---|
| Citrobacterfereundi | 0 | 0 | 0 | 1 | 0 | 0 |
| E. coli | 1 | 0 | 0 | 1 | 1 | 0 |
| Enterococcus spp. | 0 | 0 | 0 | 0 | 3 | 0 |
| Klebsiella spp. | 0 | 0 | 0 | 0 | 2 | 0 |
| Proteus spp. | 0 | 0 | 1 | 0 | 0 | 0 |
| Staphylococcus aureus | 1 | 1 | 1 | 0 | 3 | 1 |
| Staphylococcus epidermidis | 2 | 2 | 4 | 1 | 3 | 0 |
| Total (29) | 4 | 3 | 6 | 3 | 12 | 1 |
Eighteen antibiotics were utilized to treat SA between 2018 and 2020, with ciprofloxacin, cefazolin, and cephalexin being the most frequently used. From 2018 to 2020, bacterial resistance to ciprofloxacin increased from 20% to 42.9%. Resistance to cefazolin also escalated during this period, with no reported antibiotic resistance in 2018, but reaching 50% by 2020. However, resistance to cephalexin decreased from 33.3% to 27.3% over these years.
5. Discussion
Septic arthritis is a medical emergency requiring immediate measures to prevent critical functional complications. Upon confirmation of the diagnosis, the patient must be hospitalized and administered intravenous antibiotics. The incidence of this infection continues to rise due to an increasing number of risk factors, including population aging, a greater number of orthopedic and other invasive procedures, and the more frequent use of immunosuppressive therapy (4).
Diagnosis has traditionally relied on clinical judgment and cytological examination of synovial fluid. While direct bacteriological examination of joint fluid after staining can be completed within a few hours, its sensitivity has been shown to be insufficient. The gold standard for diagnosis is the examination of joint fluid culture results, but this process typically takes a few days to obtain (4, 18). History, examination, and initial laboratory procedures have all proven insufficient for reliably establishing a diagnosis of SA, with a positive synovial fluid specimen obtained through aspiration or intra-operative specimen collection generally required (18).
The evidence upon which to base the choice or duration of antibiotic therapy for SA is scarce, and to our knowledge, no randomized trials have been conducted. The selection of the drug depends on the Gram stain result, the patient’s age, and, in some cases, a history of sexual activity (4). Antibiotic resistance in SA can vary depending on the cause of the infection. Therefore, it is crucial to determine the appropriate antibiotic for treatment through AST to select the correct treatment regimen (19, 20).
In the present study, the majority of patients were in the age group of 52 to 79 years. In Shirani and Zarei's study, the largest number of patients were in the age group below 16 years. According to the mentioned study, 50% of patients were below 16 years old, 35% were between 16 to 50 years old, and 15% were over 50 years old (21). Talebi Taher et al.'s study yielded results similar to ours. In their study, 35% of patients were in the age group of 15 to 30 years, 13% in the age group of 30 to 45 years, 17% in the age group of 45 to 60 years, and 35% in the age group above 60 years (22).
In this study, the prevalence of infection was higher in men than in women, with a male-to-female involvement ratio of 2.6 to 1. Similar results were obtained in a study conducted by Mue et al. In their analysis, out of 30 examined patients, 60% were men, 40% were women, and the male-to-female involvement ratio was 1.5 to 1 (23). In another study conducted to investigate SA of the knee by Partezani Helito et al., the involvement rate was higher in men (60%) than in women (40%). Additionally, the average age of the patients was 41.6 years (24)
In an extensive study conducted by Barton et al. on SA in children over thirteen years, the most common joints involved were the knee (36%), hip (30%), ankle and elbow (11%), shoulder (5%), and wrist (4%) (25). In a large retrospective study conducted by Morgan et al. on 191 patients with SA in Australia over 18 years, it was found that the number of male patients was higher. Fifty-four percent had no underlying disease, and 72% were hematogenously infected. The knee in 54% of cases, and hip in 13%, were the most commonly affected joints. The cause of the disease was S. aureus in 37% of cases; Streptococcus pyogenes in 16%, and N. gonorrhoeae in 12%. They also showed that arthrotomy with antibiotic prescription was the best treatment method (26). In another study conducted by Wang et al., 16% of the cases of involvement in the lower limbs, including the knee, thigh, and ankle, were the most common involvement in the hip joint (54%) (27). In a study conducted by Sediqi et al., 56 children under five years of age diagnosed with SA were investigated. Thirty point three percent of these patients had knee involvement, 58.9% had hip involvement, and 7.7% had shoulder involvement (28).
In our study, the most common causative organism was S. epidermidis, followed by S. aureus as the most frequent. In Wang et al.'s study, the most common infectious agent was S. aureus with 43%, followed by coagulase-negative Staph with a frequency of 11%. Streptococcus pneumoniae accounted for 5%, Salmonella for 5%, H. influenzae type B for 3.3%, and Streptococcus group B for 3.3% of the other infection causes (27). The study by C. Gobao et al. investigated risk factors, screening, and treatment in patients with SA caused by S. aureus (20). Out of 215 patients with SA, S. aureus culture was positive in 64% of cases, of which 23% were MRSA. Staphylococcus coagulase-negative, group B Streptococcus, and S. pneumonia were also reported in a small number of patients. In another study by Ho Kwak et al., patients with SA who received initial treatment were compared with patients referred for the infection. In this study, the most common cause of SA was S. aureus (51.1 percent). After that, Streptococcus spp. were the most frequent one (11.1%) (29).
In the current study, 18 antibiotics were used to treat SA between 2018 and 2020, with the most common being ciprofloxacin, cefazolin, and cephalexin. During this period, bacterial resistance to ciprofloxacin increased from 20% to 42.9%, while resistance to cefazolin rose from zero to 50%. However, resistance to cephalexin decreased from 33.3% to 27.3%. In a study by Lausmann et al., promising results were obtained from antibiotic treatment using a combination of clindamycin and gentamicin. This method has been successful in eliminating the infection in the early stages and preventing infection in high-risk patients (30). In a study conducted by Weiss et al. to investigate the prevalence of methicillin-resistant S. aureus and methicillin-sensitive S. aureus in children with SA and osteomyelitis, the records of children aged 15 days to 18 years were reviewed. It was observed that in patients whose treatment regimen included vancomycin, the hospitalization time was longer compared to the group whose regimen included gentamicin and vancomycin. This study suggests that in SA and osteomyelitis, gentamicin should be considered in the initial antibiotic treatment regimen (31). Therefore, effective antibiotic treatment plays an essential role in reducing the incidence of complications of this disease.
A limitation of this retrospective study is the scarcity of information in patients' records, which prevented the discussion of underlying factors affecting the condition of the studied patients.
5.1. Conclusions
Although SA is a common disease, it is not always easy to diagnose. The critical point in treating these patients is to confirm the diagnosis and, if possible, identify the pathogen. Considering the inappropriate management of patients with SA in suspected and confirmed cases, parameters such as detailed history, physical examination, duration of treatment, correct selection of antibiotics, and the use of the proper method for determining the type of microorganism, including smear and culture of joint fluid and AST, should be considered. Our study revealed that overall bacterial resistance to ciprofloxacin and cefazolin has increased, but resistance to cephalexin decreased during two years. Therefore, early joint surgery (arthrotomy) and effective AST-based antibiotic therapy could play an essential role in reducing the infection and its complications.