1. Background
Inflammatory bowel disease (IBD) is a chronic and pathological immune inflammation of the intestines or gut, which has become increasingly prevalent worldwide in recent years. Inflammatory bowel disease encompasses several diseases such as Crohn's disease, ulcerative colitis, lymphocytic colitis, and other forms of colitis (1, 2). The etiology and pathogenesis of inflammatory bowel disease (IBD) are not fully understood, and multiple factors have been postulated, including alterations in the gut microbiome, inappropriate mucosal immune response to intestinal antigens, and changes in the innate and adaptive immune system of the gastrointestinal tract (3, 4).
A few years after the widespread use of hematopoietic and solid organ transplantation, several reports were published regarding the effect of transplantation on pre-existing intestinal diseases and the development of new post-transplant IBD, commonly referred to as de novo IBD (5-7). The incidence of IBD and other causes of intestinal inflammation after transplantation is higher in Orthotopic Liver Transplantation (OLT) patients than in the general population (8, 9). About 30% of patients with IBD experience improvement after LT, while an equal percentage experience worsening. Additionally, 14 - 30% of patients with PSC may develop de novo IBD. However, most past research has been limited to single case reports or case series involving only a few patients (10, 11). It is noteworthy that a significant number of de novo IBD cases after OLT include patients with primary sclerosing cholangitis (PSC), which is known to be associated with IBD (7, 12).
Currently, there is no clear explanation for the pathogenesis and prognosis of de novo IBD after transplantation. However, it has been suggested that infectious agents may play a more prominent role in this group of patients than in other patients with IBD (4, 13). Immunosuppressive drugs have been known to have multiple effects on the intestinal epithelium and the intestinal immune system. These effects can result in immune dysfunction and gastrointestinal inflammation in OLT patients (8, 11).
To better understand the incidence rates and common risk factors and determinants of intestinal inflammation after OLT, a retrospective study was conducted on patients who underwent transplantation in Shiraz, Iran, the largest transplantation center in the country. The study spanned eleven years.
2. Objectives
The first aim of this study was to estimate the incidence of de novo IBD in patients undergoing OLT and to categorize them into three groups: Patients with PSC, Autoimmune Hepatitis (AIH), and other patients with IBD. The second aim of the study was to identify any association between de novo IBD after OLT and factors such as type(s) of immunosuppression, serum levels of cyclosporine or tacrolimus, specific microbial/viral infections, or leukocyte counts.
3. Methods
3.1. Patient Characteristics and Study Design
This study followed a single-hospital design, involving the review of hospital records of 1 702 liver transplant patients. Conducted over eleven years, from 2001 to 2012, it took place at the Transplant Center of Shiraz University of Medical Sciences, Shiraz, Iran. Patients known to have IBD before undergoing OLT were excluded from the study. Data collection utilized a checklist, gathering variables such as age at transplantation, sex, family history of IBD, and date of death from hospital records. Additionally, data related to the status of liver transplant patients were obtained through hospital records and telephone calls to the patients.
For patients transplanted for AIH were evaluated the use status of immunosuppressive drugs at the time of transplantation, colonoscopy reports, histopathological descriptions, and laboratory parameters. The severity of IBD was not assessed according to currently accepted international criteria in this study. Patients were categorized into three groups: AIH, PSC, and other causes. Due to the strong association between PSC and IBD, the PSC patient group was excluded from the analysis. Therefore, in the bivariate analysis, two groups were considered: Patients transplanted for AIH and patients transplanted for various other causes.
The study investigated various factors, including registered colonoscopy reports post-transplantation, histopathological biopsy reports, types of immunosuppressive drugs used, presence of specific infections such as CMV, leukocyte count during the interval between transplantation and examination, colonoscopy findings, and colonic inflammation.
3.2. Inflammatory Bowel Disease Before and after OLT
Data were collected from the studied patients regarding various changes, including registered colonoscopy reports after transplantation, histopathological reports of biopsies taken, the existence of IBD before transplantation, type of IBD, time of diagnosis, extent of disease, presence of wounds during endoscopy, types of immunosuppressive drugs used, presence of specific infections including CMV, IBD activity at the time of transplantation, medication for IBD in the hospital before transplantation, number of leukocytes in the interval between transplantation and the time of examination, colonoscopy findings, and colonic surface inflammation. As part of the study, AIH patients (n1 = 10) and other liver transplant patients with IBD (n2 = 10) were selected as case group and liver transplant patients without IBD was selected as control group( n1 = 21, n2 = 21). The controls were selected from the same transplant registry used to identify the cases. For each patient, two control cases were selected from among the transplanted patients without intestinal problems.
3.3. Statistical Analysis
The data were analyzed using SPSS software, version 22. Descriptive statistics, including mean, standard deviation (SD), and frequency tables, were used for analysis. Analytical statistical methods, including the Chi-square test and t-test, were employed to compare IBD in the AIH group and the group of other liver transplant patients. The level of significance was set at 0.05.
4. Results
4.1. Descriptive Statistics
The overall incidence rate of IBD in liver transplant patients was found to be 2.35% (new IBD, n = 40). The patients were stratified into three groups: 254 patients who underwent transplantation for AIH, 211 patients for PSC, and 1 237 patients for other causes (Table 1). The mean age and time interval between OLT and diagnosis of colitis of the 10 patients who underwent transplantation for AIH were found to be 35.01 ± 13.05 years and 25 ± 16.50 months, respectively.
| Group Patients (OLT) | No (%) | IBD (No.) | Incidence Rate Per 1000 | CI 95% |
|---|---|---|---|---|
| AIH | 254 (14.92) | 10 | 39.37 | 31.12 - 46.29 |
| PSC | 211 (12.40) | 20 | 94.78 | 74.64 - 111.45 |
| Other patients | 1237 (72.38) | 10 | 8.08 | 5.80 - 12.14 |
| Total | 1702 (100) | 40 | 23.50 | 17.93 - 29.27 |
Incidence of de Novo Inflammatory Bowel Disease (IBD) in Liver Transplant Patients
4.2. Transplanted Patients for AIH Who Developed De Novo Colitis after OLT
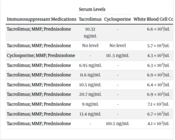
Out of the total 254 patients who underwent transplantation for AIH, 245 patients were diagnosed with ALH. The median time between the diagnosis of ALH and OLT was found to be 20.1 ± 14.41 months, with a range of 1 - 52 months. The colonoscopy indications for the patients included in the study showed that 5 patients presented with hematochezia, 2 patients with diarrhea, one patient with dysentery, one patient had chronic diarrhea, and one patient had fever and anorexia. The colonoscopy findings indicated diffuse edema and fragility, erythema and edema, erythema and fragmented edema, erosion, wounds, aphthous lesions, and fragility, all of which showed distinct wounds. The status of the transplant patients who developed de novo colitis after OLT, specifically those who underwent transplantation for AIH, is reported in Table 2.
| Immunosuppressant Medications | Serum Levels | White Blood Cell Counts | |
|---|---|---|---|
| Tacrolimus | Cyclosporine | ||
| Tacrolimus; MMF; prednisolone | 10.32 ng/mL | - | 6.6 × 103/uL |
| Tacrolimus; MMF; prednisolone | No level | No level | 5.7 × 103/uL |
| Cyclosporine; MMF; prednisolone | - | 111 .5 ng/mL | 4.3 × 103/uL |
| Tacrolimus; MMF; prednisolone | 6.95 ng/mL | - | 6.3 × 103/uL |
| Tacrolimus; MMF; prednisolone | 11.6 ng/mL | - | 6.9 × 103/uL |
| Tacrolimus; MMF; prednisolone | 10.5 ng/mL | - | 6.4 × 103/uL |
| Tacrolimus; MMF; prednisolone | 20.7 ng/mL | - | 6.9 × 103/uL |
| Tacrolimus; MMF; prednisolone | 9 ng/mL | - | 7.1 × 103/uL |
| Tacrolimus; MMF; prednisolone | 13.4 ng/mL | - | 6.7 × 103/uL |
| Tacrolimus; MMF; prednisolone | - | 101.5 ng/mL | 4.1 × 103/uL |
Mean White Blood Cell Count (WBC) and Serum Levels of Cyclosporine and Tacrolimus
The extent of colon involvement during colonoscopy was considered as follows: (1) Diffuse erythema, edema, and friability of the entire colon; (2) Normal rectum, erythema, and edema of the other parts from the sigmoid to the cecum; (3) Patchy areas of erythema and edema involving all areas from the rectum to the cecum; (4) Discrete ulcers in the rectum, erosions in the sigmoid and left colon, and normal other parts; (5) Normal rectum, aphthous-like lesions in other parts from the sigmoid to the cecum; (6) Two discrete ulcers in the ascending colon, normal other parts; (7) Normal rectum, sigmoid, descending colon; inflammation and edema of the transverse, ascending, and cecal parts; (8) Friability of the rectum, sigmoid, and descending colon; normal other parts. The terminal ileum was found to be normal grossly when intubated in the patients.
4.3. Evaluation of White Blood Cell Count (WBC) and Serum Levels of Cyclosporine and Tacrolimus
According to the information presented in Table 2, there were mean white blood cell (WBC) counts and serum levels of cyclosporine and tacrolimus, as well as immunosuppressive drugs in the AIH patients undergoing OLT, from the period immediately after transplantation to the diagnosis of colitis. The mean cyclosporine level for two patients was 106.5 ± 32.12 ng/mL, and the mean tacrolimus level for seven patients was 11.78 ± 14 ng/mL.
4.4. Transplanted Patients for Various Other Indications Who Developed Colitis after OLT
Out of 1 237 patients who were treated with OLT for various indications, 10 patients developed complications that resulted in colonoscopy and evidence of colitis. Information about these patients is presented in Table 3. The median time between diagnosis of OLT and diagnosis of colitis was 53.9 months (range: 6 - 140 months). The colonoscopy indications showed that five patients had hematochezia, two patients had diarrhea, one patient had diarrhea and pancytopenia, three patients had chronic diarrhea, one patient had fever and anorexia, and two patients had anorexia and weight loss. The underlying diseases showed that two patients had Wilson's disease, three patients had cryptogenic cirrhosis, one patient had Byler's disease, one patient had HBV cirrhosis, two patients had tyrosinemia, one patient had alcoholic cirrhosis, and one patient had non-alcoholic steatohepatitis (NASH) with HCC. The gross colonoscopic findings included diffuse edema, fragility, erythema and edema, erythema and fragmented edema, erosion, wounds, pest-like lesions, and fragility, which showed distinct wounds.
| Variables | N | Range | Average |
|---|---|---|---|
| Total number of patient | 1237 | - | - |
| Time interval between OLT and diagnosis of colitis (mon) | - | 6 - 140 | 53.9 |
| Ages (y) | - | 12 - 60 | 31 |
| Patients developed colitis | 10 | - | - |
| Incidence | 808/100 000 | ||
| Male/Female ratio | 9/1 (9) | - | - |
| Indications for Colonoscopy | |||
| Hematochezia | 5 | - | - |
| Diarrhea | 2 | - | - |
| Diarrhea and pancytopenia | 1 | - | - |
| Chronic diarrhea | 3 | ||
| Fever and anorexia | 1 | - | - |
| Anorexia and weight loss | 2 | - | - |
| Underlying Disease | |||
| Wilson disease | 2 | - | - |
| Cryptogenic cirrhosis | 3 | - | - |
| Byler disease | 1 | - | - |
| HBV cirrhosis | 1 | - | - |
| Tyrosinemia | 1 | - | - |
| Alcoholic cirrhosis | 1 | - | - |
| NASH with HCC | 1 | - | - |
Evaluation of Different Symptoms of Patients Who Develop Colitis after OLT Transplantation
4.5. Mean WBC and Serum Levels of Cyclosporine and Tacrolimus
The WBC counts during the period between transplantation and the development of colitis were calculated and presented in Table 4. The mean cyclosporine level for three patients was 107.8 ng/mL, and the mean tacrolimus level for eight patients was 8.68 ng/mL.
| Patients Who Develop Colitis After OLT Transplantation | |||
|---|---|---|---|
| Immunosuppressant Medications | Serum Levels | White Blood Cell Counts (Average: 5.2 × 103/uL) | |
| Tacrolimus | Cyclosporine | ||
| Tacrolimus; MMF; Prednisolone | No level | No level | 5.3 × 103/uL |
| Cyclosporine; Azathioprine; Prednisolone Cyclosporine; MMF; Prednisolone | - | 59 ng/mL | 4.8 × 103/uL |
| MMF; Cyclosporine; PrednisoloneMMF, Tacrolimus Sirolimus | 7.22 ng/mL | 125 ng/mL | 7.1 × 103/uL |
| Tacrolimus; Prednisolone | 10.2 ng/mL | - | 4.9 × 103/uL |
| Tacrolimus; MMF | 9.6 ng/mL | - | 6.1 × 103/uL |
| Tacrolimus | 11.56 ng/mL | - | 4.2 × 103/uL |
| Tacrolimus; MMF | 6.15 ng/mL | - | 5.1 × 103/uL |
| Tacrolimus; MMF; Prednisolone | 11 ng/mL | - | 6.2 × 103/uL |
| Cyclosporine; MMF; Prednisolone | 4.37 ng/mL | 139.5 ng/mL | 3.8 × 103/uL |
| Tacrolimus; Prednisolone | 8.87 ng/mL | - | 5.2 × 103/uL |
Comparison of White Blood Cell Count (WBC) and Serum Levels of Cyclosporine and Tacrolimus
4.6. Controls
The control group consisted of 40 patients, divided into two groups: 19 with AIH and 21 with other diseases. The demographic features, immunosuppressant medications, their serum levels, and the WBC counts of the control patients have been presented. The control patients with AIH were 12 females and 7 males (M/F = 0.58). The mean age of these patients at the time of OLT was 26.6 years, with a range of 8 to 57 years. The average follow-up time for these patients was 84 months, with a range of 57 to 112 months (as shown in Table 5).
| Variables | N | Range | Average |
|---|---|---|---|
| Total number of patient | 19 | - | - |
| Sex | |||
| Male | 7 | ||
| Female | 12 | ||
| Age (y) | - | 8 - 57 | 26.6 |
| Follow-up time of the patients (mon) | - | 57 - 112 | 84 |
| Immunosuppressant medications | |||
| Tacrolimus, MMF, prednisolone | 14 | - | - |
| MMF; prednisolone | 3 | - | - |
| Cyclosporine; MMF; prednisolone | 2 | - | - |
Characteristics of AIH patients in the control group
4.7. Risk Factor Analysis
There were no statistically significant differences between cases and controls regarding age, mean leukocyte counts, types of immunosuppressant medications, and serum levels of cyclosporine and tacrolimus in both the AIH group and the other patient groups. However, IBD developed earlier in patients with AIH (group 1) compared to other patients (group 3), with a median time of 20.1 months vs. 53.9 months. The sex ratio in group 3 was 9 (OR = 2.71; CI 95%: 0.53 - 12.37). Furthermore, there were no statistically significant differences in terms of age between cases and the control group in both the AIH patients (OR = 1.25, CI 95%: 0.86 - 1.78) and the patients with other outcomes (OR = 1.41, CI 95%: 0.81 - 1.82). The mean leukocyte counts, types of immunosuppressant medications, and serum levels of cyclosporine and tacrolimus were also not significant in the case and control group. In the group with other diseases (group 3), there were no significant differences between those who developed colitis and those who did not in terms of age, gender, WBC count, and calcineurin inhibitor serum levels.
5. Discussion
This study is unique in that it includes the largest number of patients who developed IBD after OLT and separates them into three groups. These groups include patients with PSC who developed IBD after OLT, patients with AIH and IBD after transplantation, and transplant patients for other causes of liver damage complicated by colitis. Regarding the AIH group, statistical analysis showed that age and gender did not show any notable disparity between cases and controls. Additionally, there were no significant differences in WBC counts and serum levels of calcineurin inhibitors.
The overall incidence of IBD in the study was found to be 2.35%, which is higher than that reported in other studies. Several factors may have contributed to this variation, including differences in study populations, transplant protocols, follow-up periods, and diagnostic periods among different studies. Furthermore, patient characteristics, such as the etiology of the disease, immunosuppressive regimens, and genetic factors, may also contribute to the development of IBD once it has been established (12-14).
In our study, the average time interval between transplantation and diagnosis of colitis was significantly shorter in the AIH group (20.1 months) compared to the other causes group (53.9 months). One important limitation of this study is the absence of documents showing CMV infection of the gastrointestinal tract in patients who developed colitis after OLT. This is an important masquerader of IBD and could not be confirmed or ruled out in the patients, which limits the conclusions that can be drawn from this study. The most important conclusion of this work is the high incidence of colon inflammation after OLT in the patients compared to previous studies. The incidence was 2 362/100 000/year in the AIH group, 179/100 000/year in other patients transplanted excluding PSC and AIH, and 522/100 000/year in all patients. This finding highlights the importance of monitoring patients who have undergone liver transplantation for the development of colitis.
This series represents the largest number of patients investigated for de novo IBD after OLT, rendering it more reliable than previous studies. It surpasses even the largest study conducted by Shepela at the University of Minnesota (13). The extensive sample size and meticulous data analysis in this study offer valuable insights into the incidence and characteristics of colon inflammation after OLT in patients with various causes of liver failure (15).
Based on the results, there were no significant differences in age, leukocyte counts, immunosuppressant medications, and serum levels of cyclosporine and tacrolimus between cases and controls in both the AIH and other patient groups. However, patients with AIH developed IBD at an earlier stage than other patients. In the group with other diseases, there were no significant differences between those who developed colitis and those who did not in terms of age, gender, WBC count, and calcineurin inhibitor serum levels. This finding is partially consistent with past studies (11, 15, 16).
According to the results, patients with de novo IBD after OLT exhibited a range of symptoms and signs, including fever, bloody diarrhea, and chronic diarrhea. Gross colonoscopic findings showed aphthous ulcers, discrete ulcers, patchy ulcers, mucosal erythema and edema, erosions, and diffuse erythema. Microscopic examination of biopsies taken from the mucosa of the colon of patients with abnormal colonoscopies revealed various histopathologic changes, including chronic colitis with moderate activity, cryptitis, crypt abscess, edema, and congestion, crypt disorganization, focal active colitis, apoptotic cells in crypt epithelium, lymph-plasma cell, and eosinophilic infiltration. There were no specific gross or microscopic finding differences between patients with AIH and others who developed colitis after OLT. Possible mechanisms of colon inflammation after OLT include CMV infection and perhaps other infective agents, which can simulate IBD both in clinical and pathologic presentations (17). There is a growing concern that post-transplantation colitis may be caused by colon infection among patients who are undergoing immunosuppression. Healthcare providers need to conduct appropriate laboratory investigations before diagnosing these patients with de novo IBD cases after transplantation. Additionally, the term 'dysbiosis' is used to refer to a change in the microbiome that can lead to a predisposition to diseases such as Crohn's disease and ulcerative colitis. Immunosuppressive agents may cause dysbiosis and alter the controlled inflammation in the body, leading to inflammation (16). It has been observed that certain immunosuppressive medications, such as MMF, can cause damage to the intestinal epithelium. This damage can lead to disruption of the gut's innate immune system, particularly the epithelial layer, and trigger an inflammatory response through various mechanisms such as apoptosis pathways or exposure to microbial antigens (14). Calcineurin inhibitors work by inhibiting the proliferation and activation of helper T cells, ultimately resulting in a decrease in the production of interleukin-2 (IL-2).
In the thymus, it prevents autoimmune processes by promoting the differentiation of certain immature T cells into regulatory T cells, which suppress other T cells that are otherwise primed to attack normal healthy cells in the body (15, 18). IL-2 enhances activation-induced cell death. It also stimulates naive CD4+ T cell differentiation into Th1 and Th2 lymphocytes while impeding differentiation into T17 and follicular Th lymphocytes. IL-2 increases the cell-killing activity of both natural killer cells and cytotoxic T cells (16, 19). By blocking the IL-2 effect, calcineurin inhibitors can promote autoimmune damage by interfering with tolerance mechanisms (20).
Suppression of anti-inflammatory macrophages and IL-10 production and effect, by corticosteroids and calcineurin inhibitors (18, 19).
Macrophages play a key role in various steps of inflammation, including identification, reaction, and resolution (21). According to their function, they are categorized as inflammatory, wound-healing, and regulatory/anti-inflammatory (22).
The inflammatory macrophages are triggered by IFNγ released by NK and T-helper1 (Th1) cells and TNFα from antigen-presenting cells (18). After activation, they generate significant amounts of pro-inflammatory molecules such as TNFα, IL-12, and IL-6, as well as reactive oxygen and nitrogen compounds. These agents promote the activity of Th1 and Th17 cells while inhibiting the production of the anti-inflammatory cytokine IL-10 (23). The inflammatory macrophages are essential in the reaction to infections within cells; however, they have the potential to worsen IBD as they generate pro-inflammatory cytokines (20).
The wound-healing macrophages are activated by IL-4 secreted from granulocytes and Th2 cells (22). These cells produce higher levels of IL-10 and lower levels of pro-inflammatory cytokines, and by inhibiting NLRP3 inflammasome activation, contribute to wound healing, and facilitate angiogenesis, tissue remodeling, and removal of debris (19, 24). Wound-healing macrophages have shown a protective role in murine models of intestinal inflammation, with a possible contribution to fibrosis in CD (25).
Regulatory or anti-inflammatory macrophages have been characterized recently. They are triggered by macrophage-derived TGFβ, IL-10, or immune complexes along with a pro-inflammatory stimulus (26). In addition, T-cells are activated by the expression of costimulatory molecules by regulatory macrophages (27). Contrary to wound-healing macrophages, they are unable to produce extracellular matrix. Regulatory macrophages are crucial in subsiding the inflammatory reaction by decreasing the production of IL-12 and do not promote fibrosis (26, 28).
In the intestinal lamina propria, macrophages play a critical role in maintaining balance by actively dealing with infectious agents through phagocytic and microbicidal actions, while simultaneously fostering immune tolerance towards commensal microorganisms. IL-10 and TFGβ in the surrounding milieu help stimulate the development of macrophages into a tolerant phenotype (28, 29).
Resident macrophages, unlike circulating blood monocytes, do not demonstrate an oxidative burst or inflammatory response. However, chemokines attract circulating blood monocytes to inflammatory sites in the intestinal epithelium, thereby exacerbating the disease (30). The intimate interactions between α4β7 and MAdCAM-1, along with other adhesion molecules and cadherins, facilitate the invasion of blood monocytes into nearby tissues.
The development of severe acute or chronic inflammation in the intestines leads to an increase in pro-inflammatory blood monocytes. These monocytes then transform into inflammatory macrophages, aggravating the clinical and pathological condition (30). The oxidative burst activity and production of pro-inflammatory cytokines are exaggerated in macrophages taken from patients with IBD (29). Nevertheless, the invading monocytes can suppress the inflammatory reaction by releasing IL-10.
5.1. Conclusions
Based on the results, it can be concluded that colon inflammation is more prevalent after liver transplantation than previously reported. It can occur in all patients transplanted with various causes of liver failure. However, in this series of patients, the colitis was not as severe as classic IBD, and no specific gross or microscopic stigmata were seen to distinguish it from idiopathic IBD. Future studies can investigate possible mechanisms of inflammation to delineate the pathogenesis of IBD in all patients, including those who develop de novo IBD after solid organ transplantation. Overall, the findings suggest that clinicians should be aware of the possibility of colitis in patients who have undergone liver transplantation and should monitor them accordingly.