1. Background
Self-tolerance failure to various autoantigens is implicated in autoimmunity. In such cases, a combination of genetic and environmental risk factors triggers abnormal activations of both innate and adaptive immune responses. Neutrophils may play a role in initiating immune deregulation and failure through the production of pro-inflammatory cytokines, direct tissue damage, and the formation of neutrophil extracellular traps (NETs), or NETosis (1). NETosis is identified as a unique form of cell death, distinct from necrosis and apoptosis. This multistep process involves the breakdown of nuclear, granular, and internal membranes, which are then extruded into the extracellular space, although the cytoplasmic membrane integrity remains intact. The contents of neutrophil granules move to the nucleus, inducing chromatin decondensation and the formation of NETs (2). Neutrophil extracellular traps comprise decondensed chromatin, histones, antimicrobial peptides such as myeloperoxidase (MPO), neutrophil elastase (NE), double-stranded (ds) DNA, high mobility group protein B1 (HMGB1), matrix metalloproteinases (MMPs), proteinase 3 (PR3), and, to a lesser extent, cathepsins, which can trap, immobilize, inactivate, kill microorganisms, and activate other immune cells (3). In NETosis, various molecules like histones, MPO, NE, and dsDNA are externalized, considered potential autoantigens that might contribute to the generation of autoimmune responses in predisposed individuals. An imbalance in NETs formation and degradation may increase the half-life of externalized molecules, producing modified autoantigens that, upon exposure to the immune system, might increase the risk of autoimmunity (1). The link between NETs formation and autoimmunity was first suggested in 2009 in patients with antineutrophil cytoplasmic antibody (ANCA) associated vasculitis (AAV) (4).
Recent studies have shown that viral infections stimulate neutrophils to produce NETs and antiviral agents, and may even trigger apoptosis in these cells. Virus-induced NETs can lead to extreme systemic hyperactivation, resulting in the production of cytokines, chemokines, immune complexes, and inflammation. Viral infections such as human T-lymphotropic virus type I (HTLV-I), cytomegalovirus (CMV), hepatitis C virus (HCV), Epstein-Barr virus (EBV), and human immunodeficiency virus (HIV) are associated with autoimmunity (5). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, in particular, induces excessive NET formation and immune system hyperstimulation. Multiple autoimmune diseases, including antiphospholipid syndrome (APS), systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), Guillain-Barre syndrome (GBS), and Kawasaki disease (KD) have been reported in coronavirus disease 2019 (COVID-19) patients (6). Autoimmunity may occur in viral infections such as SARS-CoV-2, with NETs and associated proteins serving as significant sources of autoantigens. These autoantigens have previously been reported as potential causes of hyperinflammation and autoimmunity.
2. Objectives
This study aimed to investigate NETosis and autoimmunity in COVID-19.
3. Methods
To enhance our understanding of NETosis in autoimmune diseases and COVID-19, we searched the PubMed database using the keywords: Autoimmune disease OR Autoimmunity AND SARS-CoV-2 OR COVID-19 AND NETosis OR NETs. Ultimately, 55 articles were selected for review.
4. Neutrophil Extracellular Trap Formation
To date, three main pathways of NETs formation have been identified. The main differences between them are the trigger stimulator, timing, and the mechanisms of NETs releasing. The first one is suicidal NETosis that takes 2 - 20 hours (7). This form of NETosis is mediated by the recognition of stimuli such as viruses, bacteria, and fungi via neutrophil receptors (e.g., IgG-Fc receptors, TLRs, and complement receptors). After neutrophil attachment to the agents, neutrophils are stimulated and NADPH oxidase assembles into a functional complex and accelerates an oxidase-dependent cellular death process that stimulates the mass production of reactive oxygen species (ROS). Reactive oxygen species accelerates suicidal NETosis and has an important role in the nuclear membrane breakdown and activation of peptidyl arginine deiminase type IV (PAD4). Peptidyl arginine deiminases, specially PAD4, leads to histone deamination and contributes to chromatin decondensation. Further chromatin unfolding promotion contributes to neutrophil azurophilic granules including NE and MPO that are released and integrate into the nucleus (7). The second form, i.e. vital NETosis, lasts 10 - 60 minutes and has been described to be a rapid release of NETs. This form of NETosis is induced by the recognition of stimuli through, C3 complement receptor, TLRs, and binding of glycoprotein Ib (GP1b) in platelets with β2 integrin. In vital NETosis, NETs are formed without breakdown of nuclear or plasma membrane and do not depend on ROS production. In the third form, i.e. mitochondrial NETosis, mitochondrial DNA is released and depending on ROS production, it lasts within 15 - 20 minutes of neutrophils stimulation with C5a or lipopolysaccharide (7).
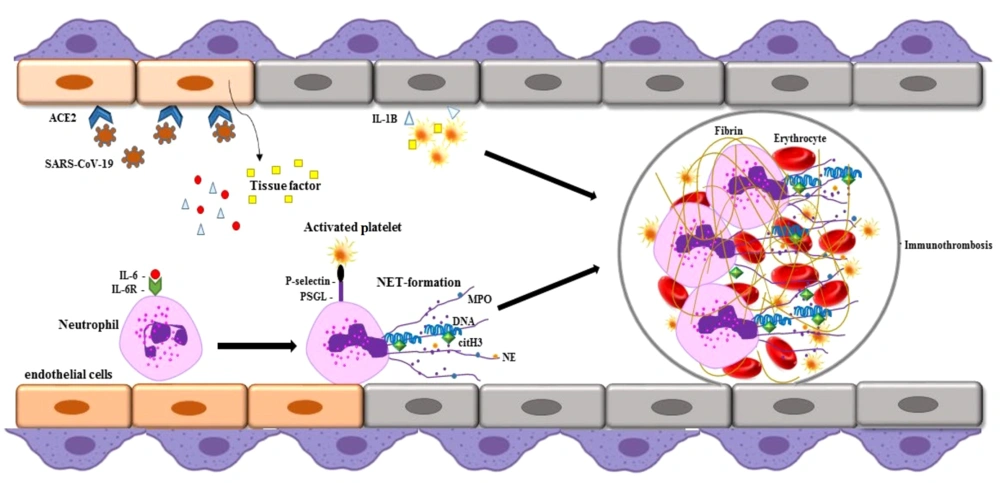
5. Significance of NETs Formation in COVID-19
Neutrophil extracellular traps may contribute to autoantigen formation in autoimmune diseases. Excessive NET formation has been observed in active autoimmune disorders, such as SLE, APS, RA, KD, ITP, and AIHA (6). Neutrophil extracellular traps-derived azurophilic granules, including NE and MPO, release PADs that increase the citrullination of proteins such as histones and cartilage proteins, potentially initiating pathogenic inflammatory cascades in these diseases. Elevated NET levels have also been reported in the sera of COVID-19 patients compared to healthy individuals. Overproduction of NETs and the release of neutrophil-mediated pro-inflammatory cytokines are linked to COVID-19 pathogenesis (6) (Figure 1). Clinical studies have noted significant neutrophilia in patients who have died from COVID-19 compared to survivors. In these patients, activated neutrophils undergo degranulation, releasing procoagulant NETs and inflammatory mediators that may exacerbate coagulation system activation and inflammatory lung tissue damage, potentially leading to severe and fatal disease complications (8). COVID-19 is associated with a range of autoimmune diseases, sharing common pathophysiology through NET formation. This suggests an irregular innate immune response to viral pathogens in autoimmune diseases. A likely mechanism for autoimmunity development in COVID-19 patients is the virus's ability to over-activate the immune system, over-release NETs, and the molecular mimicry between viral antigens and host components (8). Research indicates that the prevalence of COVID-19 is nearly double in autoimmune patients compared to the general population (9). More than 15 types of autoimmune diseases, such as APS, GBS, KD, AIHA, ITP, SLE, polyneuritis cranialis, thyroid disorders, Graves’ disease, vasculitis, viral arthritis, myasthenia gravis, and type 1 diabetes have been reported in COVID-19 patients (6). Additionally, olfactory alterations such as anosmia or hyposmia can occur, similar to autoimmune diseases like SLE and myasthenia gravis. In viral infections, the virus may enter the CNS through the olfactory bulb, causing acute inflammation and demyelination of the nervous system. These olfactory changes may also have an autoimmune basis in SARS-CoV-2 infection (10). Collectively, these findings underscore that NETosis and hyperinflammation are central to the pathogenesis of severe COVID-19, highlighting the need to evaluate NETosis activity in patients and determine if it influences the clinical progression of the disease.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) binds to the ACE-2 receptor and enters the host cells. During coronavirus disease 2019 (COVID-19) infection, neutrophil extracellular trap (NET) formation occurs and can lead to dysfunction in vascular endothelial cells. Neutrophil extracellular traps contain decondensed chromatin, histones, antimicrobial peptides such as myeloperoxidase (MPO), neutrophil elastase (NE), and citrullinated histones that may be involved in COVID-19 autoimmunity.
6. Autoantibodies in COVID-19 Patients
Some viral infections can induce autoimmunity. In HIV, HTLV-I, and HCV infections, various autoantibodies such as anti-nuclear (ANA), anti-double-stranded DNA (anti-dsDNA), anti-Smith (anti-Sm), anti-Ro52, anti-Ro60, anti-nucleosome, and anti-histone (anti-H2A/H4) may be detected (11). SARS-CoV-2 infection can lead to the production of multiple types of autoantibodies, such as anti-phospholipid antibodies (aPLs), anticardiolipin (aCL), and lupus anticoagulant (LAC), which in many severe cases result in thrombosis and coagulation dysfunction. In severe patients, anti-Ro52 (20%) and anti-Ro60 (25%) antibodies can be detected, associated with SLE and subacute cutaneous lupus (SCLE) (6). These antibodies bind to cell surface membrane proteins, inducing cell destruction and activation of the coagulation cascade. Both cytoplasmic-antineutrophilic autoantibody (c-ANCA) and perinuclear-antineutrophilic autoantibody (p-ANCA) were reported in critically ill COVID-19 patients (6). These findings support the notion that SARS-CoV-2 can overstimulate the immune system and promote the synthesis of multiple autoantibodies.
7. Role of NETs in Various Autoimmune Diseases and SARS-CoV-2 Infection
7.1. Systemic Lupus Erythematosus and APS
High levels of NETs have been detected in the skin and kidneys of patients with SLE. These NETs contain autoantigens and immune-stimulating proteins including IL-17 and IL-37 (12). In these patients, the degradation of NETs is hindered due to the production of DNase1 inhibitors or NET-binding autoantibodies that can prevent DNase1's access to NETs, resulting in prolonged presence of NETs. This prolonged exposure of the immune system to NETs might accelerate autoimmunity, pro-inflammatory responses, and tissue damage. In patients, NETs may induce plasmacytoid dendritic cells (pDCs) to produce high titers of type I IFNs (12). Additionally, NET-related proteins in SLE can lead to the production of IL-1β and IL-18, further amplifying inflammatory responses. Neutrophil extracellular trap components are also potential mediators of high-density lipoprotein (HDL) oxidation and endothelial injury, leading to atherosclerotic cardiovascular disease in these patients (13). NETosis has been implicated in the development of post-COVID-19 syndrome. Case reports have shown a connection between SARS-CoV-2 and SLE. Inflammation and tissue damage caused by SLE, as well as the use of glucocorticoids and other immunosuppressive therapies, could be risk factors for developing severe COVID-19 (14). Several cases of SLE have been reported following COVID-19 vaccination. COVID-19 has been found to effectively stimulate pDCs, leading to robust IFN-I induction. Type I IFNs may play a crucial pathological role in the development of various autoimmune disorders, including SLE, following COVID-19 vaccination (15).
Antiphospholipid syndrome is associated with viral infections and elevated titers of aPL, including aCL, anti-β2GP1, and LAC, which are implicated in venous/arterial thrombosis and miscarriages (16). In APS, neutrophils are prone to spontaneously releasing NETs, and a high level of circulating NETs is detectable, promoting thrombosis. Additionally, NETs are a primary source of tissue factor (TF), leading to platelet activation, aggregation, and the formation of atherosclerosis and arterial thrombosis. In APS, neutrophils appear to have increased adhesion potential to the endothelium (16). Like in SLE and SLE/APS, NETs in APS stimulate pDCs to produce high titers of type I IFNs and enhance autoimmunity (16). High levels of NET components, including cfDNA and MPO-DNA complexes, have been observed in the sera and plasma of primary APS patients. The decreased ability to degrade NETs contributes to the pathogenesis of APS. Similar to SLE, the degradation and clearance of NETs in APS patients are likely hindered by the presence of autoantibodies (17).
Currently, there is limited information on the role and significance of aPL in COVID-19, and evidence suggests that aPLs may have minimal clinical association with prolonged activated partial-thromboplastin time (aPTT) and thrombosis in COVID-19 patients (18). Studies have reported moderate/low aPL titers in COVID-19 patients. These antibodies primarily target β2GP1 but exhibit epitope characteristics different from those in APS. However, there are reports of clinical similarities between thrombotic events and thrombocytopenia in recipients of the COVID-19 vaccine and APS (18). Adenoviral vector-based vaccines are thought to contain epitopes that could induce aPL synthesis due to their molecular mimicry mechanism. In contrast, mRNA-based COVID-19 vaccines have demonstrated high tolerability and immunogenicity in APS patients, with no reported thrombotic events or severe complications in these patients (19).
7.2. Immune Thrombocytopenic Purpura and Autoimmune Hemolytic Anemia
ITP can be idiopathic or triggered by a viral infection. In ITP, autoantibodies target GP IIb-IIIa and/or GPIb-IX on the platelet surface, leading to platelet activation and consumption (20). Activated platelets bind to immobilized neutrophils, and platelet-neutrophil interactions result in hyperstimulated neutrophils and NET release. The histones in NETs can activate platelets and enhance thrombin production via TLR-2 and TLR-4. Additionally, NETs bind to fibrinogen and von Willebrand factor, promoting the activation of coagulation factor XII. Platelet microparticles (MPs), which are elevated in patients and have high thrombogenic potential, contribute to NET formation (21). Significant levels of ICAM-1, thrombomodulin, and citrullinated histone H3 (H3Cit; a NETs marker) were found in patients' sera. Excessive NET formation plays a significant role in thrombosis and contributes to hypercoagulability in patients. Moderate thrombocytopenia was observed in one-third of COVID-19 patients, primarily at the infection's onset. The hyperinflammatory state during the infection can significantly activate platelets and induce deadly clot formation (22). Bleeding and a sharp drop in platelet count were reported in cases of ITP, making monitoring platelet counts essential (23). Cases of ITP have been reported following COVID-19 vaccinations with Pfizer and Moderna. Managing ITP treatment is challenging during infection or following COVID-19 vaccinations (24).
Autoimmune hemolytic anemia is associated with many infections, particularly viral ones. In this condition, antibody attachment to the RBC membrane increases the likelihood of phosphatidylserine (PS) exposure. The destruction of the RBC membrane releases MPs carrying negatively charged PS, which bind tightly to vascular endothelium, leading to thrombin formation and intravascular thrombosis (25). Moreover, heme-mediated NET formation plays a major role in AIHA pathogenesis. Free heme has pro-inflammatory effects on endothelial cells and directly activates neutrophils, triggering NET release through ROS signaling. The NETs induced by heme can activate and damage endothelial cells, contributing to venous thromboembolism (VTE) (25). C5a and C3 components of the complement system are involved in neutrophil recruitment and NET formation. C1q prevents NET degradation by directly inhibiting DNase-I, thus amplifying and propagating NETs (26). Several cases of AIHA have been reported in COVID-19 patients. Ankyrin-1 protein on the RBC membrane has an immunogenic antigenic epitope that may act as a molecular mimicry agent, potentially triggering AIHA in COVID-19 patients (27). Several severe cases of AIHA following COVID-19 vaccination are being investigated as potential side effects of the vaccination (28).
7.3. Kawasaki Disease and GBS
Kawasaki disease, which typically appears after viral infections, activates the immune system, causing inflammation and damage to blood vessels, particularly in individuals genetically predisposed to the disease. It is the most prevalent childhood vasculitis and the leading cause of acquired heart diseases. Neutrophil extracellular traps play various roles in KD pathogenesis, with their formation increasing during the acute phase of KD (29). This increase in NETosis is driven by high levels of pro-inflammatory cytokines, including TNF-α, IFN-γ, IL-8, and IL-1β, which prime neutrophils to spontaneously form NETs. During the acute phase, NETs significantly inhibit neutrophil apoptosis, enhancing neutrophil survival. The delayed apoptosis of neutrophils may contribute to their increased numbers in the bloodstream (29). Circulating neutrophils undergoing NETosis activate pro-inflammatory macrophages to produce macrophage extracellular traps (METs). The presence of NETs and METs in the bloodstream predisposes patients to thrombosis and vasculitis. Overproduction of NETs also generates ANCA, associated with vasculitis in patients (30). Neutrophil extracellular traps can also activate the PI3K/Akt and NF-kB signaling pathways, leading to HIF-1α upregulation, intensifying inflammatory responses, and impacting cell survival (31).
At least 14 viral strains have been linked to KD. A significant increase in cases was observed during the influenza A H1N1 pandemic and a similar peak during the COVID-19 pandemic, suggesting a potential connection between the infection and KD (32). Several cases of KD in children with current or previous SARS-CoV-2 infection have been reported. The WHO has referred to the condition as multisystem inflammatory syndrome in children (MIS-C), with infrequent cases in adults known as MIS-A (33). Given the low COVID-19 prevalence among children, those with KD should be carefully screened for the infection. While there is evidence of a significant link between KD and COVID-19, according to AHA Kawasaki guidelines, all patients met one or two of the 5 required criteria (33). Furthermore, several cases of MIS-A have also been reported following COVID-19 vaccination (34).
Guillain-Barre syndrome is known as a rare but very serious autoimmune disorder of unknown origin, generally triggered by various infections. Molecular mimicry has been identified as a major mechanism for the disease. In response to specific antigens, immune cells attack the myelin sheath of peripheral nerve cells, leading to muscle weakness and possibly paralysis. In patients, MMPs, as part of NETs, have the ability to degrade myelin basic proteins and are associated with the production of pro-inflammatory cytokines (35). It has been reported that neutrophil-lymphocyte ratio (NLR) levels in the sera of patients are higher than in healthy individuals. Neutrophil-lymphocyte ratio levels are positively correlated with disease severity and the rate of NETs formation (36). Several studies have reported a substantial increase in the number of activated T cells in the peripheral blood as well as changes in regulatory T cells (Treg) (37). Increased H3Cit may inhibit gene-specific chromatin-mediated silencing in T cells, resulting in elevated levels of inflammatory cytokines derived from T cells, which may play a crucial role in the disease pathogenesis (38).
Nearly 30% of COVID-19 patients experience neurological symptoms, and there are reports on the disease's impact on the nervous system and the development of GBS. SARS-CoV-2 binds to the angiotensin II receptor through its spike protein. This receptor is abundantly found on the cell membranes of organs such as the lungs, liver, kidneys, skeletal muscle, and heart, as well as in neurovascular endothelium and olfactory epithelium, and myelin-forming cells (oligodendrocytes) (39). All of this supports the possible relationship between COVID-19 and GBS, suggesting that COVID-19 may trigger GBS and exacerbate its symptoms, a condition that has been recently associated with other bacterial or viral infections, including Campylobacter jejuni, EBV, CMV, Haemophilus influenza, and Zika virus (40). Acute polyneuropathy (a common form of GBS), Miller Fisher syndrome, and polyneuritis cranialis (rare subtypes of GBS) have been observed in some COVID-19 patients. Moreover, GBS with COVID-19 should be distinguished from other critical neuropathy and myopathy illnesses (41). The mechanism of GBS development in patients has not yet been fully investigated, and it is unknown whether SARS-CoV-2 causes the production of antibodies against gangliosides. Several reports of GBS have emerged after COVID-19 vaccination (42), but it is unclear whether previous vaccinations contributed to the development of GBS. As a result, more studies are needed to clarify the mechanism of GBS development in patients and the relationship between GBS and COVID-19 vaccination.
7.4. Rheumatoid Arthritis
Rheumatoid arthritis is characterized by inflammation in the synovial joints and the generation of anti-citrulline protein antigens (ACPA). Increased NET formation has been observed in the plasma, synovial fluid, synovial tissue, and skin of patients, and NET levels are positively correlated with ACPA levels (43). Circulating citrullinated antigens, predominantly derived from NETs, can stimulate the immune system. Significant levels of inflammatory cytokines, including IL-8, IL-17A, and TNF-α, stimulate neutrophils to form NETs in these patients. Additionally, reactivity against citrullinated histones, primarily H2A and H2B, has been identified in approximately 40% of monoclonal antibodies in rheumatoid synovial tissue (43). During NETosis, the citrullination of exposed proteins is carried out by PAD, especially PAD4. Histones present in NETs play a critical role in vascular damage and increase the risk of cardiovascular disease, putting patients at a higher risk (44). In RA, levels of NET-associated MPO-DNA complexes are linked with increased peripheral blood neutrophil counts and positivity for rheumatoid factor (RF) and ACPA. Neutrophil elastase and MPO may trigger the release of active PADs, enhancing the likelihood of autoantibody production. Autoantibodies, citrullinated histones, and NET components (including nucleosomes, cfDNA, NE, and MPO) are elevated in patients and may serve as new biomarkers for diagnosing the disease (43). Evidence suggests that respiratory viral infections can be associated with the onset of RA. Therefore, SARS-CoV-2 may potentially be involved in the development or recurrence of RA. Rheumatoid arthritis patients have a higher risk of contracting COVID-19 compared to non-RA patients (45). Those treated with DMARDs and prednisone are at the highest risk of developing severe COVID-19. It is estimated that RA is associated with a 25% and 35% increased risk of contracting the infection and of hospitalization or death from COVID-19, respectively (46). Evidence has shown the occurrence of RA autoimmunity following COVID-19 vaccinations (47).
8. NETosis Inhibition as a Therapeutic Target in Autoimmune Diseases
NETosis plays a critical role in the pathogenesis and exacerbation of various autoimmune diseases because NETs contain autoantigens that can stimulate the host immune system to induce autoimmunity and amplify inflammatory responses (3). Targeting NET formation might be a valuable therapeutic strategy to prevent autoimmunity and improve clinical outcomes in COVID-19 infection. Previous reports have suggested that increased NETs may serve as a biomarker of disease activity in autoimmune diseases. Currently, targeting NETs is a focal point in developing therapies for SLE and may be applicable to other autoimmune diseases. Antimalarials and novel agents, such as N-acetyl-cysteine (NAC), DNase I, PAD inhibitors, and anti-IFN-α therapies, target NETs in SLE. Although anti-IFN-α therapies have had some success in phase I and II trials, these therapies may be particularly beneficial for the subset of SLE patients with elevated type I IFN levels (48). In various autoimmune diseases, anti-IL-1β therapy prevents the activation and accumulation of neutrophils and subsequently NET formation. The recombinant anakinra protein, an IL-1β receptor antagonist, is a potential target for COVID-19 treatment and is currently undergoing clinical trials (https://clinicaltrials.gov: NCT04324021, NCT04330638, NCT02735707). Other potential inhibitors of the NET formation process, such as NE, PAD4, and gasdermin D protein (GSDMD) during inflammation, are being considered as therapeutic approaches. Additionally, glucocorticoids, as inhibitors within the NETosis axis, can block neutrophils' function and prevent NET formation. Furthermore, exogenous DNase treatment could improve NET clearance, and recombinant human DNase I is currently under investigation for safety and efficacy in clinical trials for COVID-19 (49).
9. Conclusions
Substantial evidence highlights the role of NETs in the pathogenesis of sepsis. During sepsis, platelets are activated and lead to the activation of neutrophils in a TLR4-dependent manner, resulting in the formation of procoagulant NETs (50). Tissue factor-bearing NETs contribute to coagulation activation in sepsis patients. Platelets bind to neutrophils via the PSGL-1 receptor and P-selectin, inducing NET formation in septic mice. Excessive formation of NETs during sepsis can induce multiple organ dysfunction and systemic inflammation, increasing the risk of thromboembolism and disseminated intravascular coagulation (DIC) (50). Sepsis patients exhibit elevated levels of NET components like cfDNA, which leads to impaired fibrinolytic activity and enhanced thrombosis (51). Thrombocytopenia during sepsis is likely due to the binding of activated platelets to NETs. Sepsis often results in acute lung injury (ALI), with abundant NETs observed in the ALI mouse model (52). In sepsis, extracellular histones, particularly H3 and H4, exhibit cytotoxic properties against endothelial cells and contribute to mortality in septic mice. Citrullinated histone and PAD4 are recognized as markers of sepsis severity (53).
Current evidence closely associates COVID-19 with sepsis and septic shock, which are leading causes of death in critically ill ICU patients. A severe cytokine storm plays a crucial role in the pathogenesis of COVID-19 (54). Cultured specimens from septic COVID-19 patients have shown that approximately 80% had no bacterial or fungal infections, indicating that viral infection was the primary cause of sepsis (55). Identifying molecular and clinical mechanisms in the pathogenesis of sepsis-associated COVID-19 is crucial for improving treatment outcomes in severely ill patients. While NETs play a significant role in host defense against pathogens, their overproduction can lead to the failure of self-tolerance and immune system activation, contributing to autoimmunity and autoimmune diseases observed in many COVID-19 patients. Autoimmunity in COVID-19 shares common pathophysiology with other viral infections through NET formation. Targeting NETs in developing therapies for SLE is currently a critical focus that may also be beneficial for other autoimmune diseases. Agents such as NAC, DNase I, and PAD inhibitors target NETs in SLE (48). Other potential inhibitors of NET formation, such as NE and GSDMD during inflammation, represent additional therapeutic approaches. Glucocorticoids, as inhibitors within the NETosis axis, can block neutrophil function and prevent NET formation. Additionally, DNase I therapy at the time of admission in severe cases could improve NET disruption, offering a valuable therapeutic strategy to prevent autoimmunity in COVID-19 infection.