1. Background
Globally, human beings have faced unprecedented epidemics of deadly viruses since the beginning of the 21st century, including Zika, Ebola, SARS, and MERS. Either pre-existing viruses evolved or new viral species emerged, causing outbreaks of these viral diseases. Such diseases have caused significant damage to societies. Subsequently, in December 2019, the world faced a new virus known as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused the Coronavirus disease 2019 (COVID-19) pandemic, one of the most important health challenges of this century (1). SARS-CoV-2 is a member of the Coronaviridae family, which consists of large, enveloped, single-stranded RNA viruses. Coronaviridae is divided into two subfamilies, Coronavirinae and Torovirinae. The Coronavirinae subfamily includes four genera: Alpha-, beta-, gamma-, and delta-coronaviruses. While most coronaviruses cause mild respiratory or enteric infections, certain members of this family, like the coronavirus responsible for severe acute respiratory syndrome (SARS) and the coronavirus associated with Middle East respiratory syndrome (MERS), have the potential to induce severe respiratory illness (2-4).
COVID-19, a highly contagious infectious disease, has had a catastrophic impact on the world's demographics, causing the deaths of more than 6 million people worldwide, making it the most significant global health crisis since the 1918 influenza pandemic (5). In Iran, more than 7 million cases and more than 140,000 deaths due to this disease have been reported so far (6).
SARS-CoV-2 is primarily transmitted through respiratory droplets and close contact (7, 8). The initial symptoms of COVID-19 involve fever, cough, myalgia, fatigue, or dyspnea. In advanced stages, dyspnea can progress to acute respiratory distress syndrome (ARDS) or multiple organ dysfunction (9). CT scans of patients often show bilateral pneumonia with a ground-glass pattern, which is a characteristic diagnostic sign of the disease (10-12).
The immune system plays a significant role in the pathogenesis of COVID-19, with inflammatory cytokines, particularly TNF, IL-2, and IL-6, being produced. The cytokine storm, characterized by an excessive immune response, is one of the leading causes of death in COVID-19 patients (9, 13). The cytokine storm has also been reported to be associated with the exacerbation of many infectious diseases, including SARS (14) and MERS (15).
Both cellular and humoral immune responses are involved in combating SARS-CoV-2. Monocytes and NK cells are essential in cellular immunity and increase in number during the acute phase of infection. In humoral immunity, IgM is produced in the acute phase of the disease (7 to 9 days after the onset of symptoms), followed by IgG antibodies after approximately 14 days. IgM levels decrease drastically over time, while IgG remains in the blood for a more extended period and can confer immunity. The more severe the disease, the higher the amount of antibodies produced (16, 17). Diagnosing the acute and chronic stages of COVID-19 is possible by measuring IgG, IgM, and IgA antibody levels. Demey et al. found that the presence of anti-SARS-CoV-2 IgM and IgG antibodies enables rapid and convenient detection of COVID-19 (18).
In a comprehensive study of healthcare workers in Wuhan, China, 4.39% of asymptomatic individuals tested positive for SARS-CoV-2 using ELISA while working as asymptomatic carriers (19). Another study in China reported a 10.1% prevalence of asymptomatic carriers among healthcare workers (20).
Seroepidemiological studies provide estimates of SARS-CoV-2 infection rates. Estimating the point prevalence of infection is valuable for managing public health measures during the reopening of social and economic activities. Given that healthcare workers are at the forefront of the fight against COVID-19, they likely represent a significant portion of affected cases in society. Thus, measuring specific SARS-CoV-2 antibodies in healthcare workers is important to identify asymptomatic carriers, prevent disease spread, assess immunity, and determine when they can safely return to work.
2. Objectives
This study aims to investigate the seroepidemiology of COVID-19 in healthcare workers at Dezful Ganjavian Hospital.
3. Methods
3.1. Study Design, Population, and Sampling
In this descriptive cross-sectional study, we assessed the prevalence of SARS-CoV-2 infection among asymptomatic active healthcare workers by utilizing serological testing to detect the presence of anti-SARS-CoV-2 antibodies. This approach allowed us to evaluate the extent of SARS-CoV-2 infection in the target population and gain insights into the potential transmission dynamics within the hospital setting. The included sections were ICU, Internal Medicine, Emergency Room, Radiology, Operating Room, Labor, Laboratory, and Reception, as well as administrative staff. The study was conducted from January to March 2021. All participants provided informed consent and completed a self-report questionnaire covering demographics, medical history, recent COVID-19-related exposure and symptoms, and occupational data.
3.2. Procedures
The study included a total of 276 participants. Following completion of the questionnaire, a laboratory technician collected 5 mL of venous blood from each participant. Maintaining the cold chain, the blood samples were sent to the laboratory on the same day for centrifugation and blood serum separation. In the laboratory, the collected blood samples were subjected to centrifugation at 3000 rpm for 5 minutes to separate the serum. The separated serum samples were subsequently stored at -20 degrees Celsius until the ELISA assay was performed.
Iran’s Food and Drug Administration approved SARS-CoV-2 ELISA kits (Pishtaz Teb Diagnostics, Tehran, Iran; PT-SARS-COV-2. IgM-96 and PT-SARS-COV-2. IgG-96) (Cat. NO: 7224-96) were used to detect SARS-CoV-2-specific IgG and IgM antibodies in serum samples. The manufacturer provided the following sensitivity and specificity values for the ELISA kits: 94.1% sensitivity and 98.3% specificity for the SARS-CoV-2 IgG ELISA kit, and 79.4% sensitivity and 97.3% specificity for the SARS-CoV-2 IgM ELISA kit. The ELISA procedure followed the instructions provided by the manufacturer. A negative result indicates the absence of detectable levels of IgG antibodies against SARS-CoV-2, while a positive result indicates the presence of detectable levels of IgG antibodies against SARS-CoV-2.
3.3. Statistical Analysis
In this study, we conducted an analysis of cross-sectional data to assess the seroprevalence of SARS-CoV-2. Quantitative variables were characterized by reporting the mean and standard deviation, while qualitative variables were presented as numbers and percentages. We described continuous variables using the median and interquartile range, and categorical variables were presented as counts and percentages. To estimate the seroprevalence of SARS-CoV-2, we calculated the ratio of seropositive samples to the total number of samples tested.
We employed statistical tests, including the Mann-Whitney U test, chi-square test, and Fisher's exact test, to examine the association between participant characteristics and the presence of IgM or IgG antibodies, as well as a history of COVID-19. Additionally, binary logistic regression was used to estimate the odds ratios and 95% confidence intervals for the association between COVID-19 history and the seroprevalence of anti-SARS-CoV-2 antibodies. All statistical analyses were performed using SPSS version 20 software (SPSS Inc., Chicago, IL, USA). A significance level of 0.05 was used to determine statistical significance, with a P-value less than 0.05 considered statistically significant.
4. Results
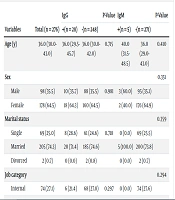
A total of 276 asymptomatic healthcare workers, with a majority of females (n = 178 [64.5%]), were included in this study. The median age of participants was 36 years (range 30 - 43), with the minimum age being 20 and the maximum age 60. Ten (3.6%) participants reported a history of autoimmune diseases, 12 (4.3%) had a history of corticosteroid administration, and 16 (5.8%) had underlying conditions (diabetes mellitus, high blood pressure, obesity, cardiovascular diseases, Hashimoto's disease, gout, chronic respiratory diseases, allergies, and thalassemia minor). Ninety (32.6%) participants had a history of SARS-CoV-2 infection within the past 1 to 9 months (Table 1).
| Variables | Total (n = 276) | IgG | P-Value | IgM | P-Value | ||
|---|---|---|---|---|---|---|---|
| +(n = 28) | -(n = 248) | +(n = 5) | -(n = 271) | ||||
| Age (y) | 36.0 (30.0-43.0) | 36.0 (29.5-45.7) | 36.0 (30.0-42.0) | 0.715 | 40.0 (31.5-48.5) | 36.0 (29.0-43.0) | 0.410 |
| Sex | 0.351 | ||||||
| Male | 98 (35.5) | 10 (35.7) | 88 (35.5) | 0.981 | 3 (60.0) | 95 (35.1) | |
| Female | 178 (64.5) | 18 (64.3) | 160 (64.5) | 2 (40.0) | 176 (64.9) | ||
| Marital status | 0.359 | ||||||
| Single | 69 (25.0) | 8 (28.6) | 61 (24.6) | 0.718 | 0 (0.0) | 69 (25.5) | |
| Married | 205 (74.3) | 20 (71.4) | 185 (74.6) | 5 (100.0) | 200 (73.8) | ||
| Divorced | 2 (0.7) | 0 (0.0) | 2 (0.8) | 0 (0.0) | 2 (0.7) | ||
| Job category | 0.294 | ||||||
| Internal | 74 (27.1) | 6 (21.4) | 68 (27.8) | 0.297 | 0 (0.0) | 74 (27.6) | |
| OR | 33 (12.1) | 3 (10.7) | 30 (12.2) | 0 (0.0) | 33 (12.3) | ||
| Employee | 83 (30.4) | 6 (21.4) | 77 (31.4) | 2 (40.0) | 81 (30.2) | ||
| Lab | 20 (7.3) | 3 (10.7) | 17 (6.9) | 1 (20.0) | 19 (7.1) | ||
| ICU | 29 (10.6) | 7 (25.0) | 22 (9.0) | 1 (20.0) | 28 (10.4) | ||
| Labor | 16 (5.9) | 2 (7.1) | 14 (5.7) | 1 (20.0) | 15 (5.6) | ||
| Radiology | 10 (3.7) | 1 (3.6) | 9 (3.7) | 0 (0.0) | 10 (3.7) | ||
| ER | 8 (2.9) | 0 (0.0) | 8 (3.3) | 0 (0.0) | 8 (3.0) | ||
| Corticosteroid | 1.000 | ||||||
| Yes | 12 (4.3) | 0 (0.0) | 12 (4.8) | 0.618 | 0 (0.0) | 12 (4.4) | |
| No | 264 (95.7) | 28 (100.0) | 236 (95.2) | 5 (100.0) | 259 (95.6) | ||
| Autoimmune | 1.000 | ||||||
| Yes | 10 (3.6) | 0 (0.0) | 10 (4.0) | 0.606 | 0 (0.0) | 10 (3.7) | |
| No | 266 (96.4) | 28 (100.0) | 238 (96.0) | 5 (100.0) | 261 (96.3) | ||
| COVID-19 vaccination | 1.000 | ||||||
| Yes | 11 (4.0) | 1 (3.6) | 10 (4.0) | 1.000 | 0 (0.0) | 11 (4.1) | |
| No | 265 (96.0) | 27 (96.4) | 238 (96.0) | 5 (100.0) | 260 (95.9) | ||
| COVID-19 history | 0.003 | ||||||
| Yes | 90 (32.6) | 20 (71.4) | 70 (28.2) | <0.001 | 5 (100.0) | 85 (31.4) | |
| No | 186 (67.4) | 8 (28.6) | 178 (71.8) | 0 (0.0) | 186 (68.6) | ||
| Exposure | 1.000 | ||||||
| Yes | 12 (4.3) | 1 (3.6) | 11 (4.4) | 1.000 | 0 (0.0) | 12 (4.4) | |
| No | 264 (95.7) | 27 (96.4) | 237 (95.6) | 5 (100.0) | 259 (95.6) | ||
| Background | 1.000 | ||||||
| Yes | 16 (5.8) | 1 (3.6) | 15 (6.0) | 1.000 | 0 (0.0) | 16 (5.9) | |
| No | 260 (94.2) | 27 (96.4) | 233 (94.0) | 5 (100.0) | 255 (94.1) | ||
Association of Characteristics of Study Participants with IgM or IgG a
We found no statistically significant relationship between a history of SARS-CoV-2 infection and variables such as sex, age, corticosteroid administration, autoimmune disease, and exposure to COVID-19 patients (P-value ≥ 0.05). However, there was a significant relationship between the participants' history of COVID-19 and underlying conditions (P-value = 0.038) (Table 2). Measurement of serum levels of IgG and IgM anti-SARS-CoV-2 antibodies showed that 28 (10.1%) and 5 (1.8%) participants were seropositive, respectively. Twenty (71.4%) of the IgG-positive individuals had a history of previous SARS-CoV-2 infection (P-value < 0.001), and all 5 (100%) IgM-positive individuals had a history of previous infection (P-value = 0.003). This indicates a significant relationship between the serum level of anti-SARS-CoV-2 antibodies and the history of COVID-19 infection.
| Variables | Total (n = 276) | COVID-19 History | P-Value | |
|---|---|---|---|---|
| Yes (n = 90) | No (n = 186) | |||
| Age (y) | 36.0 (30.0-43.0) | 36.0 (31.00 - 45.2) | 36.5 (28.7 - 42.2) | 0.223 |
| Sex | 0.583 | |||
| Male | 98 (35.5) | 34 (37.8) | 64 (34.4) | |
| Female | 178 (64.5) | 56 (62.2) | 122 (65.6) | |
| Job category | 0.060 | |||
| Internal | 74 (27.1) | 23 (26.1) | 51 (27.6) | |
| OR | 33 (12.1) | 12 (13.6) | 21 (11.4) | |
| Employee | 83 (30.4) | 23 (26.1) | 60 (32.4) | |
| Lab | 20 (7.3) | 8 (9.1) | 12 (6.5) | |
| ICU | 29 (10.6) | 15 (17.0) | 14 (7.6) | |
| Labor | 16 (5.9) | 6 (6.8) | 10 (5.4) | |
| Radiology | 10 (3.7) | 0 (0.0) | 10 (5.4) | |
| ER | 8 (2.9) | 1 (1.1) | 7 (3.8) | |
| Corticosteroid | 0.214 | |||
| Yes | 12 (4.3) | 6 (6.7) | 6 (3.2) | |
| No | 264 (95.7) | 84 (93.3) | 180 (96.8) | |
| Autoimmune | 0.084 | |||
| Yes | 10 (3.6) | 6 (6.7) | 4 (2.2) | |
| No | 266 (96.4) | 84 (93.3) | 182 (97.8) | |
| Exposure | 0.535 | |||
| Yes | 12 (4.3) | 5 (5.6) | 7 (3.8) | |
| No | 264 (95.7) | 85 (94.4) | 179 (96.2) | |
| Background | 0.038 | |||
| Yes | 16 (5.8) | 9 (10.0) | 7 (3.8) | |
| No | 260 (94.2) | 81 (90.0) | 179 (96.2) | |
Association of Characteristics of Study Participants with COVID-19 Histoty a
In contrast, no significant relationship was found between job category (workplace) and the serum levels of anti-SARS-CoV-2 IgG and IgM (P-value > 0.05) (Table 1). Additionally, this study demonstrated that there was no statistically significant relationship between job category and COVID-19 history (P-value = 0.060) (Table 2). The prevalence of serum levels of specific antibodies against SARS-CoV-2 and their significance levels based on the studied variables are shown in Table 1. Compared to the reference group (no history of COVID-19), participants with a past history of COVID-19 were significantly more likely to have a positive IgM or IgG anti-SARS-CoV-2 antibody test (OR = 6.35, CI = 2.67-15.10, P < 0.0001) (Table 3).
| Variable | β(SE) | OR (95% CI) | P-Value | |
|---|---|---|---|---|
| COVID-19 history | Yes | 1.85 (0.44) | 6.35 (2.67 - 15.10) | < 0.0001 |
| No | Reference | |||
Odds Ratio (95% CIs) for COVID-19 History Associated to the Seroprevalence of Anti-SARS-COV-2 Antibodies
Participants were also queried about recent COVID-19-related symptoms, revealing various signs in a total of 86 individuals with a history of COVID-19. Of these, the highest and lowest percentages of clinical symptoms were related to myalgia (52 participants, 18.8%) and anosmia (4 participants, 1.5%). Other symptoms included headache (48 participants, 17.4%), fever (42 participants, 15.2%), cough (40 participants, 14.5%), dyspnea (30 participants, 10.9%), and sore throat (13 participants, 4.7%).
5. Discussion
During the COVID-19 pandemic, healthcare systems faced immense pressure due to the lack of effective treatment interventions for infected patients in various hospital departments (21). Protecting healthcare workers, who are at the forefront of the fight against infection, is crucial to ensure continuous and sustainable public health and treatment services. This study aimed to identify specific antibodies against SARS-CoV-2 in asymptomatic healthcare workers and investigate their relationship with various factors. A total of 276 cases from different departments of Dezful Ganjavian Hospital were included in the study between January and March 2021.
The findings of this study indicate a high transmission rate of the disease among healthcare workers who had constant contact with patients. This can be attributed to the basic reproductive number (R0) mentioned by Li et al. in their 2020 study (22). The R0 index reflects the contagiousness of an infectious disease. According to the report, the R0 for SARS-CoV-2 was 2.2, meaning that, on average, each infected person could transmit the infection to 2.2 others (23). Therefore, healthcare workers in hospitals are at a higher risk of contracting the disease due to the inherent nature of their job.
The burden of COVID-19 on healthcare workers has been investigated in several studies worldwide. In Spain, the prevalence of anti-SARS-CoV-2 antibodies among healthcare workers was reported to exceed 8%, higher than the overall country's prevalence of 5% (24). A hospital in Belgium reported a seroprevalence of 6.4% among healthcare workers using rapid cassette testing (25). In Turkish hospitals, a study showed a seroprevalence of 2.7% among healthcare workers, with cleaning staff having the highest prevalence at 6%. Seropositive healthcare workers in ICU also had a prevalence of 4.3%. In the same study, 1% seroprevalence was reported in radiology technicians (21).
In our study, the seroprevalence of IgG and IgM anti-SARS-CoV-2 antibodies was 10.1% and 1.8%, respectively. The presence of IgG antibodies in asymptomatic individuals confirms that many people with COVID-19 can unknowingly spread the disease in the community. Additionally, 32.6% of the participants in our study had a history of COVID-19. Of the IgG-positive subjects, 71.4% had a history of previous infection with SARS-CoV-2, indicating that prior infection does not provide complete protection against reinfection. Furthermore, 100% of IgM-positive subjects had a history of previous infection with COVID-19, suggesting that the presence of IgM antibodies can serve as an indicator of prior infection. However, the absence of IgM antibodies in all those with a history of previous infection may be due to the timing of antibody measurement relative to the last infection with SARS-CoV-2.
In a study conducted to investigate the seroprevalence of SARS-CoV-2 antibodies in healthcare personnel in New York City, the seroprevalence was reported as 13.7% (26). In Los Angeles and California, the seroprevalence of SARS-CoV-2 antibodies in healthcare personnel was reported to be 4.1%, which seems to be lower than that of healthcare workers in New York City (27). Another study reported the prevalence of anti-SARS-CoV-2 antibody serum levels in healthcare workers as 10.1%, which is consistent with our results, suggesting that medical staff may act as asymptomatic vectors in the spread of the disease through direct contact. Identifying asymptomatic viral spreaders in time can help prevent the virus from spreading and protect medical staff (20).
According to another study conducted in the north of Iran, Gilan province, the seroprevalence of anti-SARS-CoV-2 antibodies using the rapid test method was 22.2%, with an 8.1% (95% CI: 16.4 - 28.5%) seroprevalence among healthcare workers (28). This is very high compared to our study results. In the study conducted by Poustchi et al., the overall prevalence of seropositivity in the study population was reported as 17.1% (29).
A study conducted by Tosato et al. in Italy aimed to investigate asymptomatic healthcare professionals. Results showed that only one person tested positive in the RT-PCR test, while six tested positive for IgG antibodies. It was observed that the majority of the staff who tested positive worked in the blood collection department, which has more patient contact (30). In another study in Lombardy, Italy, the treatment staff of seven hospitals were examined serologically. A total of 3985 subjects were included in the study. Out of these, 3462 (87%) tested negative, 76 (2%) had equivocal results, and 447 (11%) tested positive for IgG antibodies. Additionally, the proportion of IgG-positive females was found to be higher than that of males. BMI did not appear to influence the frequency of IgG-positive individuals; however, it did affect the plasma concentration of IgG. Specifically, higher BMI was associated with elevated levels of IgG. The study revealed that subjects with a smoking habit made fewer antibodies against COVID-19 (31).
According to our study findings, individuals with a history of COVID-19 have a higher probability of testing positive for SARS-CoV-2 antibodies compared to those without a COVID-19 history (Table 3). Among the IgG-positive cases with a previous COVID-19 infection, no significant associations were observed with characteristics such as underlying diseases, exposure history, autoimmune diseases, history of corticosteroid administration, age, and gender (Table 3). When considering the distribution of seropositivity by occupation, the highest prevalence was observed among the service forces, with a rate of 30.4%. This finding suggests that service forces are at a higher risk of COVID-19 infection due to their continuous presence in various hospital departments, often due to staff shortages and heavy workloads.
In light of these results, it is important to implement effective measures, such as organizing training sessions, aimed at enhancing their knowledge about this disease and promoting their overall health in dealing with COVID-19. The findings highlight the importance of seroepidemiological investigations in healthcare workers, who serve as the frontline in combating COVID-19. The study emphasizes the role of serological testing, specifically measuring IgG and IgM antibody titers, for diagnosing the acute and chronic stages of COVID-19. Such testing can provide valuable insights into the prevalence of SARS-CoV-2 infection and inform public health control measures during the reopening of social and economic activities. Further research is needed to explore the long-term immunity conferred by these antibodies and their implications for ongoing surveillance and prevention strategies.
5.1. Conclusions
In conclusion, our study has several limitations, primarily its exclusive focus on monitoring healthcare workers. Therefore, it may not provide a comprehensive representation of the entire population, as a significant number of professionals in the workforce are women, and the elderly are underrepresented due to age restrictions on employment. However, our findings demonstrate the effectiveness of antibody testing in identifying individuals who have been exposed to SARS-CoV-2. This approach serves as a valuable tool for assessing the spread of the virus, particularly among asymptomatic individuals.
Furthermore, our results indicate that despite the use of protective equipment in all hospital departments, treatment staff, regardless of age and gender, are still at risk of contracting and being reinfected with COVID-19. Additionally, previous infection with this disease does not lead to a stable increase in IgM levels and does not provide protective immunity against SARS-CoV-2. It is also worth noting that a significant proportion of infected individuals, even if they test positive for IgG antibodies, remain completely asymptomatic and can contribute to the transmission of the disease within the community.