1. Background
Colorectal cancer (CRC) is one of the most common and lethal cancers worldwide, ranking third in incidence and second in cancer-related mortality (1). Despite advancements in surgical techniques and adjuvant therapies, nearly half of CRC patients experience disease recurrence within three years after resection, highlighting the urgent need for reliable prognostic markers and new therapeutic targets (2). Colorectal cancer follows a well-defined progression, beginning with dysplasia in the intestinal mucosa, advancing to polyp formation, and eventually leading to adenocarcinoma. Various risk factors contribute to its development, including genetic predisposition, age, chronic colon inflammation, high-fat diets, and tobacco use (3).
Research increasingly underscores the pivotal role of inflammation in CRC pathogenesis. Myeloperoxidase (MPO), an enzyme primarily found in neutrophils, has gained recognition for its role in both innate immune responses and inflammation-driven carcinogenesis (4). As part of the heme peroxidase superfamily, MPO contributes to the generation of reactive oxygen species (ROS) and hypochlorous acid, which are essential for pathogen defense but can also inflict considerable tissue damage (5). Dysregulated MPO release, particularly within the tumor microenvironment, may exacerbate oxidative stress and contribute to cancer progression. Indeed, studies have shown that MPO can induce extensive tissue damage when released from activated neutrophils (6).
Myeloperoxidase production is closely linked to neutrophil maturation in the bone marrow, where it is stored in azurophilic granules until neutrophil activation occurs (7). In typical immune responses, neutrophils release MPO at infection sites to combat pathogens. However, abnormal MPO release—whether due to chronic inflammation or cancer-related processes—can intensify tissue damage and create an inflammatory environment that fosters cancer progression (8). This has generated growing interest in MPO as a potential marker for inflammatory and oxidative processes in CRC (9).
2. Objectives
This study aimed to investigate serum MPO levels in CRC patients compared to healthy controls. By analyzing the relationship between MPO expression, clinical-pathological features, and patient outcomes, we seek to assess MPO’s prognostic significance in CRC and its potential as a non-invasive biomarker. This research addresses a critical gap in understanding the interaction between neutrophil-driven inflammation and CRC and aims to contribute to the advancement of personalized treatment strategies.
3. Methods
3.1. Study Design
A total of 60 participants were included in this study, divided into two groups: Thirty patients diagnosed with CRC and 30 healthy controls. Serum samples were collected from each participant and analyzed for MPO levels using a standardized assay.
3.2. Sampling
Sampling was performed on the day of patient admission, within two hours of arrival in the emergency room. A 9 cc blood sample was drawn from each participant. Biochemical parameters (using serum) and blood parameters (using EDTA plasma) were analyzed in the standard diagnostic laboratory of Dezful Hospital. Plasma samples were prepared by centrifuging the blood at 3000 g for 10 minutes at 15 - 18°C.
3.3. Examination of Myeloperoxidase Activity
Myeloperoxidase activity was assessed using the " MPO Activity Measurement" kit (KMPO96, Kiazist Life Sciences, Iran) based on the colorimetric method. After blood collection and centrifugation at 3000 g for 10 minutes, serum and plasma were separated, diluted in buffer, and stored at -80°C until analysis. Following the preparation of samples and reagents, the MPO samples were pipetted into a 96-well plate. A reactive solution was then added to each well, and the plate was incubated for 80 minutes at room temperature. Following incubation, a Stop Reagent solution was added, and optical absorption was measured at a wavelength of 405 nm.
3.4. Statistical Analysis
Statistical analysis was conducted to compare the mean MPO levels between the CRC and healthy control groups. The significance of observed differences was determined using appropriate statistical tests. Clinical response data and additional information from both the CRC and healthy control participants were analyzed using SPSS software, version 27. The results from the CRC and control groups were initially compared using the Independent t-test. A significance level of P < 0.05 was set for all statistical analyses in this study.
3.5. Ethical Considerations
This study adhered to the principles and guidelines of the Helsinki Declaration and was conducted in alignment with the ethical standards of Dezful University of Medical Sciences. Participants received comprehensive explanations about the study, and informed written consent was obtained from each participant prior to their inclusion in the study. The study was approved under the ethics code IR.DUMS.REC.1402.044.
4. Results
4.1. Patient Demographic Information
Based on the inclusion and exclusion criteria, 60 individuals were deemed eligible for the study. Participants were selected to ensure similar demographic characteristics across groups. Among the participants, 35 were men and 25 were women, with an age range of 35 to 75 years. A chi-square test comparing the number of men and women between the two groups showed no significant difference (P = 0.906). Additionally, a t-test comparing the ages of participants in each group indicated no significant difference (P = 0.415). Thus, gender and age were not confounding factors in this study (Table 1).
| Parameters | Colorectal Cancer Group (N = 30) | Healthy Control Group (N = 30) |
|---|---|---|
| Age (y) | ||
| < 35 | 5 (16.7) | 8 (26.6) |
| > 75 | 25 (83.3) | 22 (73.3) |
| Sex | ||
| Male | 16 (53.3) | 15 (50) |
| Female | 14 (46.7) | 15 (50) |
| Tumor size (cm) | ||
| < 3 | 5 (16.7) | - |
| > 3 | 25 (83.3) | - |
| Stage at diagnosis | ||
| I | 8 (26.7) | - |
| II | 8 (26.7) | - |
| III | 10 (33.3) | - |
| IV | 4 (13.3) | - |
a Values are expressed as No. (%).
4.2. Examination of Lactate Dehydrogenase and Erythrocyte Sedimentation Rate in the Samples
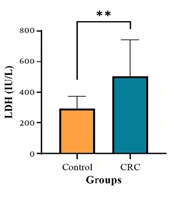
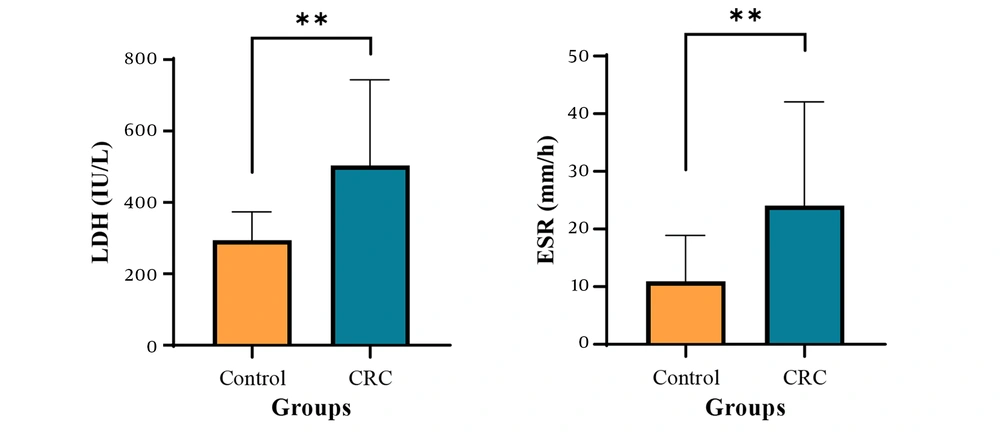
To assess the extent of cell damage in colon cancer patients, average lactate dehydrogenase (LDH) levels were compared between the patient and control groups. The results showed that the mean LDH level in the patient group (503.81 ± 239.79 IU/L) was significantly higher than in the control group (P < 0.01), suggesting extensive cellular damage in individuals with colon cancer. Elevated LDH levels may result from tumor progression, metastasis, inflammation, or damage to other organs. Additionally, to evaluate inflammation in colon cancer patients, the mean erythrocyte sedimentation rate (ESR) was compared between the groups. The findings revealed that the mean ESR in the patient group (24.06 ± 06.24 mm/h) was significantly higher than in the control group (P < 0.01), further confirming the presence of inflammation in these patient (Figure 1 and Table 2).
| Variables | Groups | P-Value | |
|---|---|---|---|
| Healthy Control (N = 30) | Colorectal Cancer (N = 30) | ||
| LDH (IU/L) | 294.29 ± 79.12 | 503.81 ± 239.79 | 0.002 |
| ESR (mm/h) | 10.94 ± 7.93 | 24.06 ± 18.01 | 0.007 |
Abbreviations: LDH, lactate dehydrogenase; ESR, erythrocyte sedimentation rate.
a Values are expressed as std. error of mean ± mean.
4.3. Examination of Myeloperoxidase Activity in the Samples
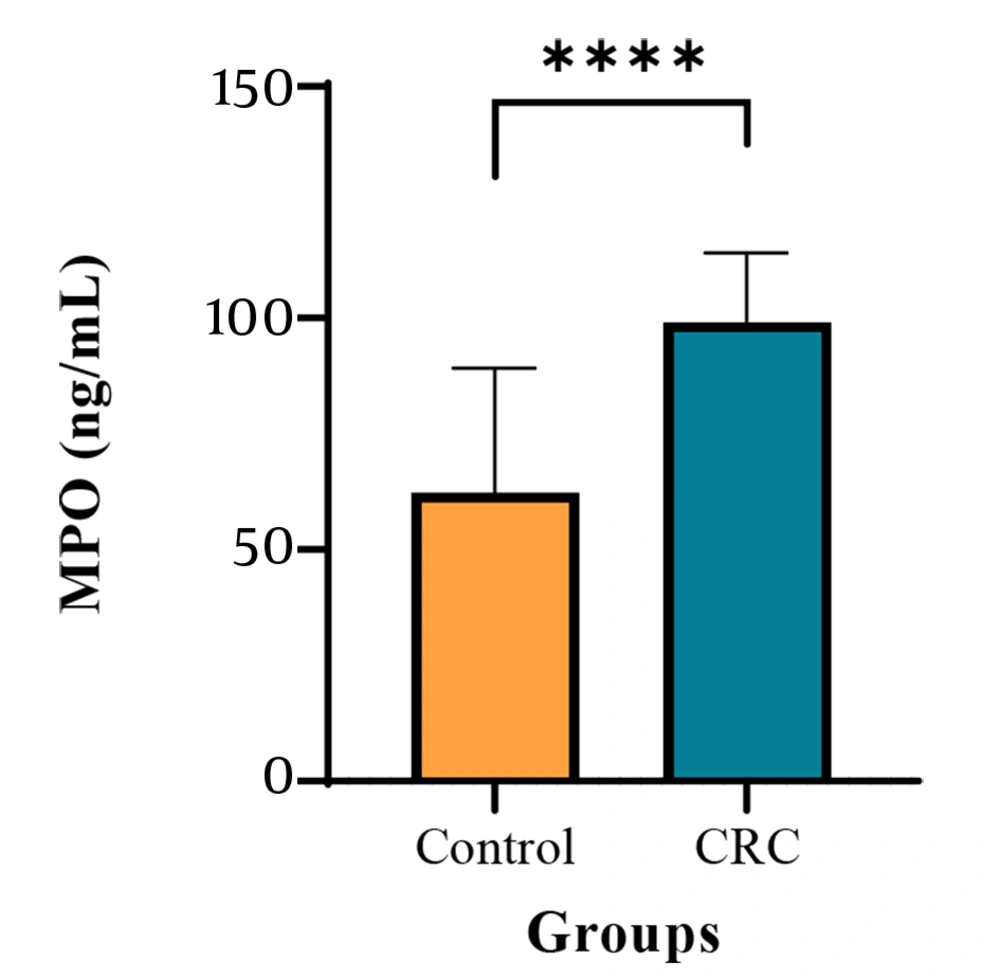
The study results demonstrated a significant increase in MPO levels in the serum of patients with CRC compared to the healthy control group. The mean MPO concentration in the CRC group was markedly elevated, suggesting a potential association between MPO levels and CRC (Figure 2).
5. Discussion
This study’s evaluation of MPO levels in CRC patients compared to healthy controls revealed a significant increase in MPO among the patients. Elevated MPO suggests heightened neutrophil activity and chronic inflammation, which are recognized as key contributors to cancer progression, including CRC (10).
Numerous studies have demonstrated that MPO, as a critical enzyme in neutrophils, plays an essential role in producing ROS (11, 12). These reactive species can damage surrounding tissues and worsen inflammation. Prior research has linked elevated MPO activity with increased ROS levels, leading to damage in both cancerous and normal cells, and potentially intensifying inflammation and tumor growth (13). For example, a study by Khan et al. found that higher MPO levels are associated with CRC progression and poorer clinical outcomes, suggesting that MPO may act not only as a marker of inflammation but also as a potential contributor to tumorigenesis (4).
The elevated MPO levels observed in CRC patients in this study align with similar findings, underscoring the enzyme’s role in tissue destruction and inflammation exacerbation (14, 15). As neutrophils respond to inflammatory stimuli, MPO release can damage both cancer cells and surrounding healthy tissues (16). However, it is important to note that elevated MPO is not exclusive to CRC and may also be present in other inflammatory conditions, posing a limitation for its use as a specific biomarker for CRC. Further research is necessary to differentiate MPO levels in CRC from those in other inflammatory conditions. Additionally, a study by Bardelcikova et al. showed that, beyond causing tissue damage, MPO may stimulate the immune system to further attack tumor cells through autoregulatory mechanisms. These mechanisms may help explain the elevated MPO levels observed in CRC patients and support MPO’s role in inflammatory processes and possibly in cancer progression (17).
The findings of this study align with previous reports linking elevated MPO levels to chronic inflammation and tissue destruction in cancer patients (18). Given that MPO acts as a local mediator of inflammation, it has been suggested as a potential therapeutic target for inflammatory diseases (19). Interestingly, while inhibiting MPO has been proposed as a strategy to reduce inflammation, research indicates that MPO deficiency can paradoxically result in an exaggerated inflammatory response (20), as MPO deficiency impairs neutrophil functions, including cytokine production, which can lead to dysregulated immune responses (21).
The role of MPO in CRC is multifaceted. While its bactericidal functions and infection control capabilities are well-documented, chronic overexpression of MPO may contribute to the persistent inflammation observed in CRC patients (22). Chronic inflammation is a recognized driver of tumor progression, promoting genetic mutations, cellular proliferation, and the survival of malignant cells (23). This dual role of MPO in immune defense and inflammation-induced damage highlights its significance as a potential biomarker and therapeutic target in CRC (14).
A limitation of this study is its relatively small sample size (n = 30 per group), which may restrict the statistical power to detect smaller differences or generalize findings to a broader population. Future studies with larger sample sizes are recommended to validate these results. Additionally, elevated MPO levels may occur in other inflammatory conditions besides CRC, suggesting that elevated MPO could result from non-cancer-related inflammation as well.
This study also explored the relationship between elevated MPO levels and increased LDH and ESR in CRC patients. Both LDH and ESR are commonly used markers of tissue damage and inflammation, respectively, and their elevation in CRC patients indicates widespread cellular injury and an active inflammatory response.
Myeloperoxidase, primarily produced by neutrophils, plays a key role in generating ROS such as hypochlorite (HOCl), which contributes to tissue damage (24). The elevated LDH levels observed in CRC patients could be associated with ROS-induced damage. Lactate dehydrogenase is an enzyme released from damaged cells, and its increase is often considered a sign of tissue breakdown (25). In CRC, elevated LDH levels may reflect tumor growth, metastasis, and the destruction of surrounding tissues—processes potentially driven by chronic inflammation and oxidative stress (26). The role of MPO in ROS generation likely intensifies these effects, contributing to higher LDH levels in CRC patients (27).
Similarly, the elevated ESR levels observed in this study indicate systemic inflammation in CRC patients. Erythrocyte sedimentation rate measures the rate at which red blood cells settle in a tube over time, typically rising in response to inflammatory processes (28). Myeloperoxidase’s role in neutrophil activation and the release of pro-inflammatory cytokines significantly contributes to sustained chronic inflammation, which is reflected by increased ESR levels (29). The link between chronic inflammation and CRC progression may explain the elevated levels of both ESR and MPO, highlighting MPO’s involvement in the inflammatory cascade in CRC patients (30).
Several studies have demonstrated a strong association between inflammation, oxidative stress, and cancer progression, particularly in CRC (17, 31, 32). In this context, MPO, LDH, and ESR are interconnected, as MPO-driven oxidative stress and inflammation lead to tissue damage (reflected by elevated LDH) and a systemic inflammatory response (indicated by increased ESR). These findings emphasize MPO’s dual role—not only as a mediator of inflammation but also as a contributor to the cellular damage and inflammatory markers characteristic of CRC.
When compared to previous studies on MPO in cancer patients, the elevated MPO levels observed here align with literature that links inflammation, oxidative stress, and cancer progression. However, some discrepancies in the degree of MPO elevation have been noted across studies, likely due to differences in patient demographics and study designs. Overall, the elevated MPO levels found in CRC patients in this study further support its association with the disease. Excessive MPO activity could contribute to CRC progression through its pro-inflammatory and tissue-damaging properties. Further research into MPO regulation in CRC could provide new insights into therapeutic strategies aimed at controlling inflammation and improving patient outcomes.
5.1. Conclusions
This study underscores MPO’s significant role as a key biomarker in CRC and its association with inflammation and tissue damage, as indicated by elevated LDH and ESR levels. The findings demonstrate that MPO is not only involved in the oxidative stress and inflammatory processes seen in CRC patients but also contributes to the disease’s progression and severity.
The increased levels of MPO, along with elevated LDH and ESR, indicate that chronic inflammation and cellular injury are central features of CRC. Myeloperoxidase-driven ROS contribute to tissue damage, while elevated LDH reflects the breakdown of tumor and surrounding cells. Additionally, increased ESR highlights the presence of systemic inflammation. Together, these markers provide a comprehensive picture of the inflammatory and pathological state of CRC patients.
Given MPO’s significance in CRC, its potential as a diagnostic and prognostic biomarker is evident. Targeting MPO-mediated pathways may also offer therapeutic benefits in managing CRC by reducing oxidative damage and inflammation. Further research is needed to clarify the exact mechanisms by which MPO influences CRC progression and to develop targeted interventions that can mitigate its harmful effects. This study underscores the need for a deeper understanding of the role of inflammation and oxidative stress in CRC, which could pave the way for personalized treatment strategies aimed at improving patient outcomes.