1. Background
The liver is one of the vital organs of the body, which is involved in regulating many physiological activities. Factors such as oxidative stress, free radicals, white alcohol, chemicals, viruses, and drugs can damage liver tissue (1). Aspartate aminotransferase (AST) and alanine aminotransferase (ALT) are intracellular aminotransferase enzymes in liver cells and are released into the bloodstream after cell death or damage to liver cells. An increase in these serum transaminase enzymes indicates a risk of liver damage (2, 3). High-intensity, long-term training can increase oxidative stress in both genders (4) by producing free radicals (5). It also might increase the serum level of aspartate aminotransferase and alanine aminotransferase (6) by decreasing antioxidant capacity (7). Moreover, superoxide dismutase (SOD), as the first defense line against the harmful effects of free oxygen radicals in the cell, prevents lipid peroxidation, and protects cells by eliminating free radicals (3).
The positive effects of low-intensity continuous training on physical function and health improvement have been proven in previous studies. However, given the research findings, When we discuss the importance of public health as well as the lack of time for people,, high-intensity interval training (HIIT) is superior to continuous training (8). It is stated that HIIT has beneficial effects on health and the championship. Comparing these two types of training regimen indicated HIIT superiority over high-intensity continuous training (HICT), and it produced more desired results in a shorter time (8, 9). Additionally, another reason for the more widespread use of HIIT is to achieve better results in less time (10). However, there is concern that HIIT can cause physiological stress and over-training symptoms due to its high intensity (11). However, the results of studies on the effects of continuous and interval training on liver damage are negligible and contradictory. For example, after 6 and 12 weeks of continuous and interval training in elderly rats, Barzegarzadeh et al. observed a significant increase in the level of alanine aminotransferase and aspartate aminotransferase (12). In a study conducted by Rezaei et al., three training sessions on a treadmill with negative slope in male rats also caused a significant increase in the serum levels of alanine aminotransferase and aspartate aminotransferase (13). In the study conducted by Mirdar et al., it was found that increasing exhausting training for one week had a significant effect on increased aspartate aminotransferase in active girls (14). However, Pettersson et al. observed that weightlifting resulted in profound increases in the liver function parameters, AST and ALT, as well as LD, CK, and myoglobin levels in healthy men (15). Therefore, it seems that physical activity not only has beneficial effects on the body but also might have negative effects under hard training conditions. Given that the use of HIIT is growing (8, 9, 16), the safety of this type of training on the liver should be ensured.
2. Objectives
Therefore, given the effects of various methods and training intensities on the production of free radicals (4), the effect of free radicals on liver cell damage and increased release of liver enzymes in the blood (3), the role of superoxide in neutralizing free radicals and protecting cells(3) , the very high intensity of HIIT, and the fewer number of relevant studies, the objective of this study was to evaluate the effects of HIIT compared to those of HICT on damage and antioxidant indices of male Wistar rats.
3. Methods
3.1. Animals
In this experimental study, 22 adult male Wistar rats aged eight weeks were purchased from the Pasteur Institute (Iran) and transmitted to the Applied Sports Physiology Laboratory of Zanjan University. The rats were randomly divided into three groups of control (n = 6), HICT (n = 8), and HIIT (n = 8) after two weeks of familiarization with the training protocol. The rats were kept in polycarbonate cages at 22 ± 2ºC in a 12-h light/12-h dark cycle and even, monitored humidity (45 - 55). Water and food were provided for them ad libitum. The research project was approved by the Ethics Committee of the Department of Sport Sciences of the Faculty of Humanities, Zanjan University. The HICT and HIIT protocols were implemented in five days per week (Saturdays, Sundays, Mondays, Wednesdays, Thursdays) for eight weeks and at certain hours of the day (3 to 6 p.m.) on a rodent treadmill.
3.2. High-Intensity Interval Training Protocol
After two weeks of familiarization, the high-intensity interval training protocol, including eight weeks of HIIT, was implemented on a treadmill specified for rats (Table 1). The familiarization program lasted two weeks (five sessions in a week), in which walking and running were performed at speeds of 5 to 8 meters per minute and inclines of zero for 5 to 10 min in the initial sessions. In all training sessions, five minutes of warm-up and five minutes of cool-down were implemented.
| Week | Familiarization | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|
| Speed (m/min) | 10 - 30 | 30 - 45 | 45 - 55 | 55 - 65 | 65 - 70 | 50 | 75 - 85 | 75 - 85 | 75 - 85 |
| Time (min/period) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Rest time (min) | 2 | 2 | 2 | 2 | 3 | 5 | 4 | 3 | 3 |
| Number of periods | 10 | 10 | 10 | 10 | 6 | 5 | 7 | 7 | 7 |
| Number of sessions per week | 6 | 6 | 5 | 5 | 5 | 3 | 5 | 5 | 5 |
High-Intensity Interval Training Protocol
3.3. High-Intensity Continuous Training Protocol
In this study, the HICT protocol included eight weeks of running on a treadmill after two weeks of familiarization (Table 2).
| Week | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Speed (m/min) | 10 | 20 | 20 | 25 | 15 | 30 | 30 | 35 |
| Time (min/session) | 30 | 40 | 45 | 50 | 35 | 60 | 70 | 70 |
High-Intensity Continuous Training Protocol
3.4. Blood Sampling
Blood samples were taken 48 hours after the last training session following eight hours of fasting under anesthesia condition. The blood samples were immediately transferred to special containers (falcon tubes) and centrifuged for 10 min at a speed of 3000 rpm. The resultant sera were kept at a temperature of -80ºC until measuring the factors.
3.5. Liver Enzyme Measurement
Liver enzymes were measured by the IFCC method (a method recommended by the International Federation of Clinical Chemistry and Laboratory Medicine) using Pars Azmoon Kits (Pars Azmoon kit, Pars Azmoon Inc., Tehran, Iran).
To measure the alanine transferase and aspartate aminotransferase from the serum, enzymatic kits of from Pars Azmoon Company (1-400-019, 1-400-018) were used following the method recommended by the International Federation of Chemistry Clinical and Laboratory Medicine (IFCC). This type of kit could measure the optical absorption changes up to 0.16 at 340 nm.
3.6. Superoxide Dismutase Activity Measurement
After dissection and sampling and sampling, liver tissue samples were rinsed by distilled water and dried by sterilized gauzes. Then, they were placed in micro-tubes, frozen in liquid nitrogen, and kept in a fridge at a temperature of -80ºC for laboratory activities. During measurements, 50 mg of liver tissue was placed in 2 mL of homogenous phosphate buffer solution. Then, it was placed in a homogenizer for 4 min at a low speed. The tube containing the suspension was centrifuged for 10 min at a speed of 3000 rpm. In the next phase, the supernatant was isolated, followed by adding the trichloroacetic acid solution. Then, the solution was frozen and warmed two consecutive times, and 1 cc of the thiobarbituric acid solution with a density of 6.7 g/L was added to the separated supernatant. Next, the tube was placed in boiling water for 15 min, and after cooling down, the resultant solution was used to measure the activity of SOD enzyme. Superoxide dismutase activity was measured by the Nishikimi method (17) and moderated by using the Kakar method. Each unit of superoxide dismutase activity was measured in the concentration of the enzyme required to prevent color production by 50% in one minute under the study conditions using the RANDSOD commercial kit (RANDOX Laboratories Ltd., UK) based on the enzymatic colorimetric method.
3.7. Data Analysis
Data were analyzed using SPSS 23 software. The Shapiro-Wilk test was used to ensure the normal distribution of data. A one-way analysis of variance (ANOVA) and Tukey post hoc tests were used for statistical analysis of data, given the normality of data. The error level was also calculated at a significance level of P < 0.05.
4. Results
Table 3 shows AST, ALT, and SOD values in all groups.
| Variables/Groups | Mean ± SD | F2.18 | Sig. |
|---|---|---|---|
| ALT (U/L) | 2.135 | 0.14 | |
| CO | 3.61 ± 53.75 | ||
| HIIT | 7.79 ± 60.00 | ||
| HICT | 6.42 ± 58.42 | ||
| AST (U/L) | 3.76 | 0.04 | |
| CO | 35.05 ± 179.50 | ||
| HIIT | 19.76 ± 141.33 | ||
| HICT | 20.29 ± 173.00 | ||
| SOD (U/mg protein) | 31.65 | 0.001 | |
| CO | 8.14 ± 32.70 | ||
| HIIT | 16.92 ± 76.28 | ||
| HICT | 24.33 ± 103.45 |
4.1. Serum Alanine Aminotransferase
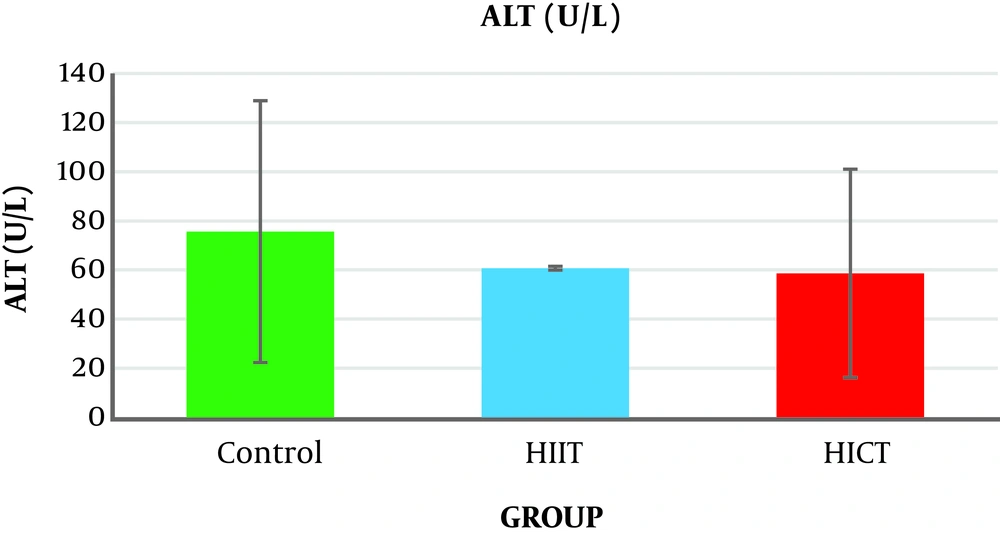
The results of the one-way analysis of variance (Table 3) showed no significant difference in the serum ALT levels between the study groups (P = 0.14, F2.18 = 2.135) (Table 3 and Figure 1).
4.2. Serum Aspartate Aminotransferase
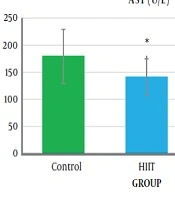
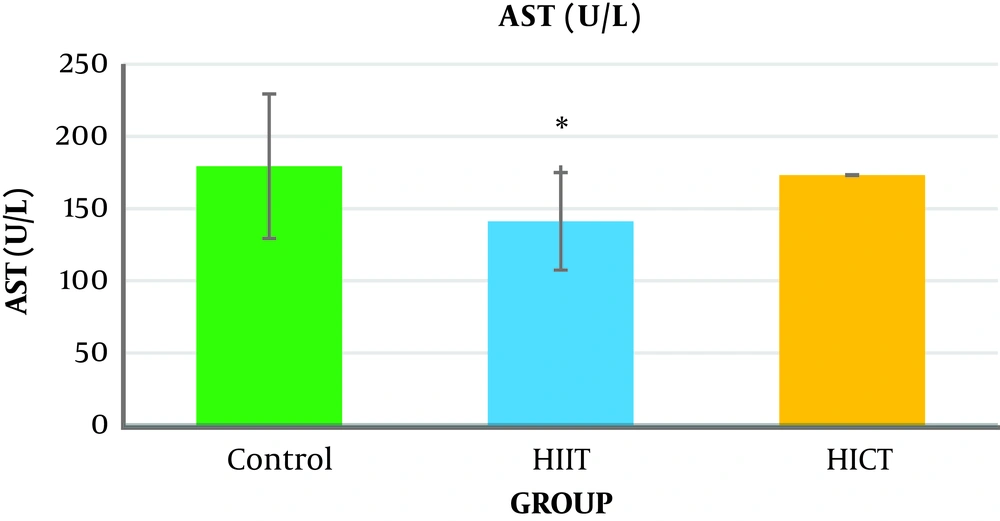
The one-way analysis of variance (Table 3) showed a significant difference in the AST levels between the study groups (P = 0.04, F2.18 = 3.76). The results of Tukey’s post hoc test showed that the serum AST level was significantly lower in the HIIT group (141.33 ± 19.76) than in the control group (971.05 ± 35.05) (P = 0.04) (Figure 2 and Table 4).
| Variables | Group | Group | Sig. |
|---|---|---|---|
| ALT (U/L) | Co | HIIT | 0.15 |
| HICT | 0.30 | ||
| HIIT | HICT | 0.88 | |
| AST (U/L) | Co | HIIT | 0.04 |
| HICT | 0.88 | ||
| HIIT | HICT | 0.11 | |
| SOD (U/mg protein) | Co | HIIT | 0.001 |
| HICT | 0.000 | ||
| HIIT | HICT | 0.03 |
The Results of the Tukey Post Hoc Test for Comparison of SOD, ALT, and AST Variables Between the Groups
4.3. Liver Tissue Superoxide Dismutase Activity
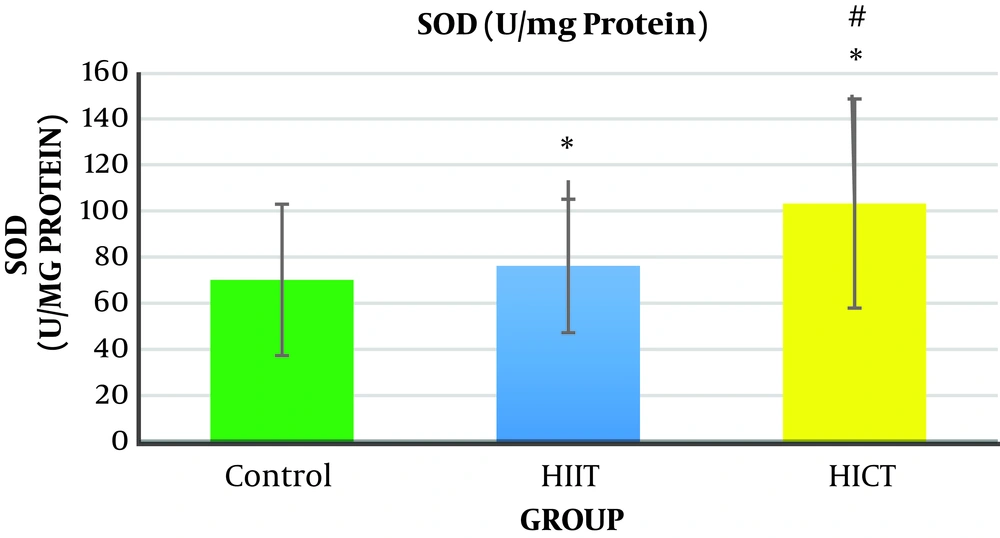
The results of one-way analysis of variance (Table 3) showed a significant difference between the study groups in liver SOD enzymatic activity (P = 0.001, F2.18 = 31.65). The Tukey post hoc test showed that the level of liver SOD enzymatic activity was significantly higher in the HICT group (103.45 ± 24.33) (P = 0.000) and HIIT group (76.28 ± 16.92) (P = 0.001) than in the control group (32.70 ± 8.14) (Table 4). Additionally, the level of liver SOD enzymatic activity was significantly higher in the HICT group (301.45 ± 24.33) than in the HIIT group (67.82 ± 16.92) (P = 0.03) (Figure 3 and Table 4).
5. Discussion
Generally, the results of the present study showed that eight weeks of HICT and HIIT had no effect on serum ALT levels, but eight weeks of HIIT significantly decreased serum AST levels compared to the control group. The level of liver SOD enzymatic activity in the HICT group and HIIT group was significantly increased compared to the control group. In addition, the SOD level was significantly increased in the HICT group compared to the HIIT group.
5.1. The Effect of HIIT and HICT on Aspartate Aminotransferase and Alanine Aminotransferase
The results showed that eight weeks of HICT and HIIT had no effect on the serum ALT levels, and eight weeks of HICT did not affect serum AST, as well. However, after eight weeks of HIIT, serum AST significantly decreased compared to the control group. These findings are not consistent with the findings of researchers such as Barzegarzadeh et al. (12), Zar et al. (18), Hoseini et al. (19), Kim et al. (20), and Rezaei et al. (13) who observed an increase in liver enzymes after an aerobic training course. For example, Barzegarzadeh et al. showed a significant increase in the level of alanine aminotransferase and aspartate aminotransferase enzymes after 6 and 12 weeks of continuous training in elderly rats (12). In their study, Kim et al. (20) also reported an increase in alanine aminotransferase and aspartate aminotransferase enzyme levels after 200 kilometers of sprint and marathon. In the study conducted by Rezaei et al., three three training sessions on treadmill with negative slope in male rats caused a significant increase in the serum levels of alanine aminotransferase and aspartate aminotransferase enzymes (13). It has been claimed that long-term endurance training increases liver enzymes, and when the activity is longer, the enzyme activity will be significantly more (21). As samples had 48 hours of rest after the end of the last training session in this study, the lack of change in the liver enzymes might be attributed to the delay in the measurement. In addition, the studies inconsistent with the present study used a shorter-term training protocol, and it seems that the intensity of training activities is an important factor in increasing the production of free radicals (4), leading to damage to liver cells and increased release of liver enzymes in the blood (3). Therefore, it seems that the intensity or duration of HICT protocol in this study was not too much to increase liver enzymes; in other words, eight weeks of training caused some kind of endogenous adaptation to prevent increased liver enzymes.
However, it should be noted that serum AST significantly decreased after eight weeks of HIIT compared to the control group, which is in line with the findings by Zar et al., who observed a decrease in the levels of aspartate aminotransferase and alanine aminotransferase in women after eight weeks of aqua gymnastics (18). Additionally, this finding is consistent with the finding by Bazegarzadeh et al., who observed lower levels of liver enzymes in interval training than in continuous training after 12 weeks (12). It has been claimed that the increase in serum levels of liver enzymes after training activity was due to liver damage and lack of adequate opportunity to return to the initial status (13). Therefore, it seems that adequate time to return to the initial status between the training courses in interval training prevents further damage to organs, including the liver, by decreasing stress (22), thereby preventing increased liver enzymes in the serum (12). It should be noted that some researchers concluded that in obese patients with fatty liver, short-term training reduced liver enzymes. However, patient’s conditions seem to be completely different from those of healthy people. Therefore, it could be stated that in HIIT, in comparison with HICT, a major part of the energy was provided through the anaerobic system, and liver cells, especially its enzymes, were not more involved in the energy production; hence, the level of damage to liver cells and the release of enzymes were lower.
5.2. Enzymatic Activity of Liver SOD
Concerning the liver SOD enzymatic activity, the results showed that liver SOD enzymatic activity was significantly increased in the HICT and HIIT groups. In addition, the level of the liver SOD enzymatic activity in the HICT group was significantly higher than that of the HIIT group. Increased activity of liver SOD in the HICT group and HIIT group was consistent with the findings of research conducted by Vani et al. (23) and Ji et al. (24), but it was inconsistent with the findings by InSurk et al. (25) and Barari et al. (26). It has been reported that by increasing the oxygen consumption by 10 to 20 times, training results in the production of intrinsic endogenous factors such as cytokines, TNF-α, corticosteroids, and adenosine, and these factors have been recognized as regulators of the activity level of antioxidant enzymes (27). Moreover, training increases the antioxidant enzyme transcription depending on the duration and intensity of the activity by increasing NF-kB (27). It seems that the increase in liver SOD enzymatic activity in both groups of HICT and HIIT is probably due to the increased oxygen consumption, increased endogenous factors such as cytokines, TNF-α, corticosteroids, and adenosine, or high intensity of both training protocols and increased lipid peroxidation. Therefore, the natural amounts of antioxidant enzymes was not enough to encounter the radicals produced following this training. Moreover, as superoxide dismutase is responsible for the removal of about 90% of free radicals produced in the body (28), its increased activity would be normal. Therefore, it seems that the duration of the training (eight weeks) did not induce the required stimuli for the adaption of the antioxidant system of superoxide dismutase in the present study. Hence, it is suggested that this issue be considered by researchers in future research.
5.3. Conclusion
As the results of the study showed that eight weeks of HIIT, compared to HICT, significantly decreased the serum AST levels and it had less effect on the increased activity of SOD in the liver tissue, it might be stated that HIIT might be safer than HICT with lower destructive effects on liver tissue.