1. Background
Toxoplasma gondii (T. gondii) is a compulsive intracellular parasite of the Apicomplexa phylum that contaminates a wide range of vertebrates, including humans, and causes disease. Around one-third of the population worldwide has a serologically positive toxoplasmosis test (1-3). The cat is the ultimate and the main host of the parasite. The oocysts of the parasite are excreted in cat feces and contaminate the environment, as well as vegetables, fruits, and water (3, 4). Therefore, health poverty is important in the transmission of the disease. Military personnel, who are involved in operations and maneuvers, are more likely to become infected than civilians (5). Despite that the disease is mild in healthy people with no or mild clinical symptoms, it can be dangerous for pregnant women who have contracted the disease for the first time and may be transmitted to the fetus (6).
Dangerous side effects in children include: microcephalus, hydrocephalus, miscarriage, etc. The disease is dangerous for immunocompromised individuals such as those treated with immunosuppressive drugs, HIV-positive patients, cancer patients, etc. (7, 8).
Today, sulfadiazine and pyrimethamine are used as first-line medications. However, these drugs have side effects (9), including bone marrow suppression. Other chemical products used to treat toxoplasmosis have little effects. Drug resistance has been reported in this parasite, like some other parasites (10). It is also failed to find a new vaccine to combat toxoplasmosis (11, 12). Nevertheless, we need medicines with less toxicity and more lethality for the parasite at all stages of the life cycle, including bradyzoites in tissues (10).
Over the last decade, the use of nanotechnology in the detection and treatment of diseases has been strongly emphasized. The construction of microcapsules, nanoparticles (NPs), conjugates, nanocomposites, etc. is one of the dimensions of nanotechnology (13). Nanosuspensions are prepared with some anti-toxoplasma drugs to compensate for their low absorption. For example, atovaquone nanosuspension has shown major impacts on the reduction of inflammation and the number of parasites in infected mice compared to non-infected mice (14-16). Today, nanoparticles are used for a multitude of biomedical applications due to their nanoscale sizes and other useful surface reactivity. In addition, NPs can create free radicals that can kill infectious agents, with the minor size of NPs allowing them to membrane barriers leading to more reactivity (17). Of particular interest for this purpose are metal nanoparticles like silver and gold, which exhibit antimicrobial activity (18), anti-parasitic activity (19), and other bioactivities, as well as the selective prevention of some enzyme activities (20).
2. Objectives
This survey aimed to assess the anti-parasitic effect of silver nanoparticles (Ag-NPs) based on ginger extract on T. gondii.
3. Methods
3.1. Synthesis of Silver Nanoparticles
Ginger-based silver NPs were synthesized by the Department of Pharmaceutical Biotechnology Research and the Research Center for Pharmaceutical Sciences, Faculty of Pharmacy, Tarbiat Modares University, Tehran.
3.2. Toxoplasma gondii RH Strain
The RH strain of T. gondii was provided by the Department of Parasitology, Tarbiat Modares University. For keeping tachyzoites and debarment of its weakness through different passages, Balb/c mice were used as pool (21). Tachyzoites were obtained in phosphate-buffered Saline (PBS; pH = 7.2) from the peritoneal fluid layer of contaminated Balb/c mice. Then, to eliminate host cells and debris, the peritoneal liquid acquired was centrifuged at 1,500 rpm for 10 min at room temperature. The pellet was then washed twice with DMEM combined with streptomycin (100 U/mL) (Gibco BRL) and penicillin (100 U/mL) (Gibco BRL). Finally, tachyzoites were duplicated in DMEM-containing Vero cells and developed in a 25 mL tissue culture jar at 37°C in a humidified 5% CO2 incubator.
3.3. Tachyzoite Assay
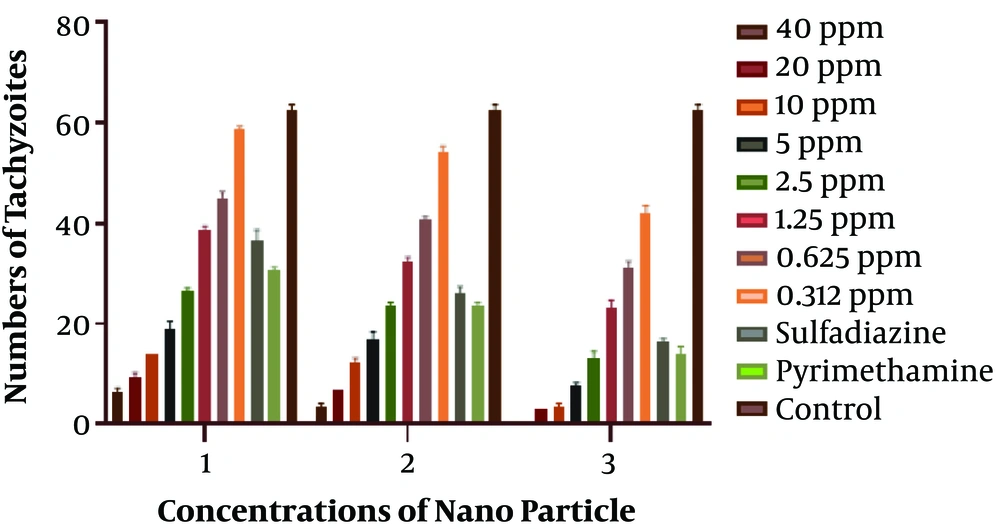
In this research, the toxic effect of silver NPs on T. gondii was investigated at eight different concentrations (40, 20, 10, 5, 2.5, 1.25, 0.625, and 0.312 ppm) in 96-well plate culture. Tachyzoites were then analyzed after three, six, and 24 hours using the trypan blue dye by light microscopy in terms of numbers and morphology, and compared with two control groups, namely sulfadiazine and pyrimethamine. It should be mentioned that all experiments in this study were conducted in triplicate.
3.4. Macrophage Viability Measurement by MTT Assay
To prepare the MTT solution (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), we mixed 5 mg of MTT powder (Gibco, German) with 1 cc of sterile PBS in dark condition. The effect of NPs on mouse macrophages was evaluated using the MTT assay. These cells were cultivated in RPMI 1640 medium (Gibco, Germany) containing 10% FBS (Gibco, Germany) with 100 µg/mL streptomycin and 100 IU/mL penicillin in a 37°C incubator with 5% CO2. They were then exposed to concentrations of 40, 20, 10, 5, 2.5, 1.25, 0.625, and 0.3122 ppm of nanoparticles in a 96-well culture plate to explore the toxicity of NPs and determine its toxic dilution. After 24 h of incubation at 37°C, the liquid was drained from the wells and 20 mL of MTT solution was added to each well. Then, the plate was incubated at 37°C for 5 h to break down the tetrazolium salts into undissolved aqueous crystals of formazan. Finally, we added 100 mL of DMSO to the wells in dark conditions to dissolve floor crystals and read the absorption coefficient of each well using an ELISA reader device at a wavelength of 540 nm (22). The percentage of living cells was calculated as follows:
Percentage of living cells (%) = (AT-AB)/(AC-AB) ×100
AT, Absorbance of exposed promastigotes to NPs; AC, absorbance of unexposed promastigotes to NPs; AB, absorbance of the blank.
3.5. Flow Cytometry Analysis
In this study, the flow cytometry technique was used to determine the possible apoptosis by double staining with annexin V-FLUOS and propidium iodide (PI) (IQ Products BV, Groningen, Netherlands). As already described, Annexin-V can be used in this technique to differentiate between early (lower right) and late (upper right) apoptosis, necrotic (PI-positive/upper left), and living cells (both annexin-V and PI negative/lower left) (23). In this study, the Annexin V-FITC apoptosis kit (Bio vision, USA) was used. For this test, we counted 2 × 105 tachyzoites with a hemocytometer slide. The concentration of 2 ppm of Ag-NPs was selected for this experiment. After 24 hours, 500 mL of the buffer from the FLOUS kit was added, and the samples were incubated on ice for 15 min. Then, 5 mL of annexin V and 5 mL of PI were added to the samples and entered the flow cytometry device. The device displayed the results as a graph. Data were checked with Flow Jo software and expressed as percentages. The cells staining with annexin V and PI were apoptotic and necrotic cells, respectively. Also, the living cells did not stain.
4. Results
4.1. Tachyzoite Assay
The number of parasites exposed to different concentrations of Ag-NPs was counted with an optical microscope after 3, 6, and 24 h. The results showed that the higher the concentration, the greater the lethal effect on the parasite. Also, the longer the exposure to NPs, the more the disappearance rate of tachyzoites. More information is shown in Figure 1. The IC50 for NPs was calculated at 2 ppm via SPSS21 software.
4.2. MTT Assay
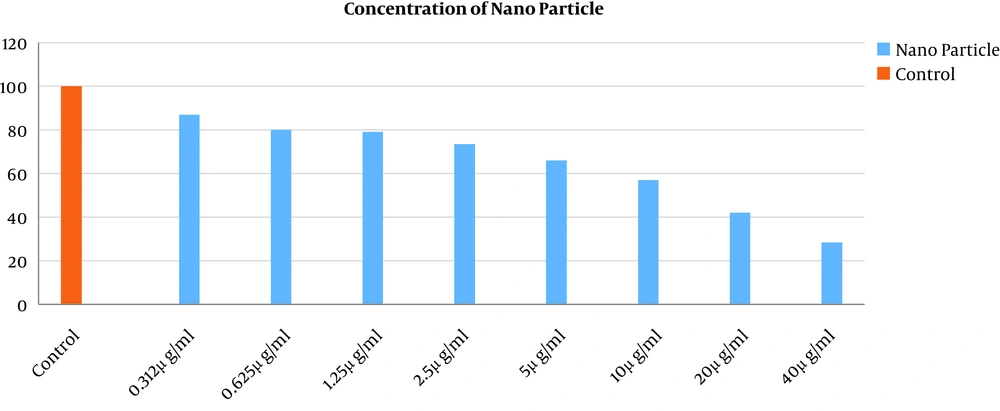
To see what concentration of NPs had a toxic effect on macrophage cells, an MTT assay was employed. The highest lethal effect on macrophage cells occurred at the NP concentration of 40 ppm (Figure 2). More than 80% of the macrophages were viable at a concentration of 0.312 ppm and there was no significant difference with the control group. More information is shown in Figure 2.
4.3. Flow Cytometry Analysis
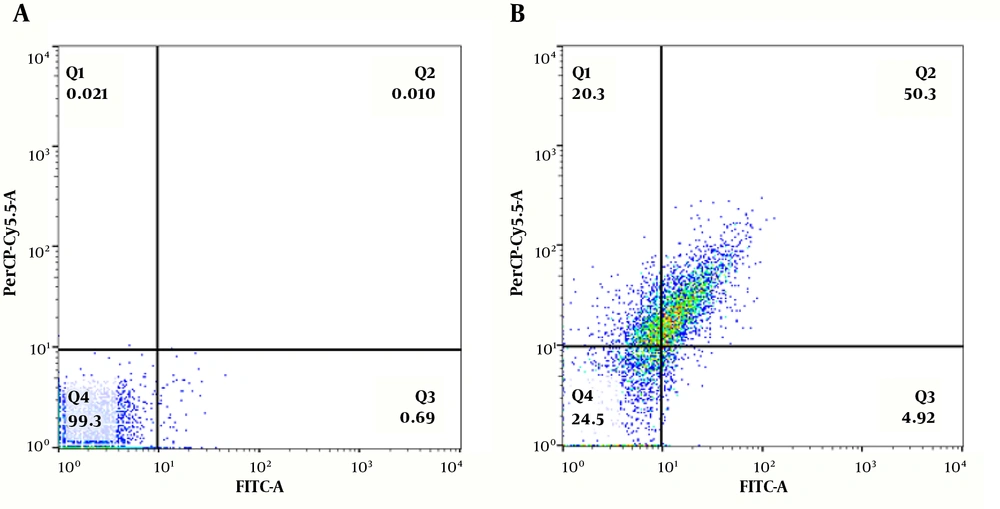
In this research, a flow cytometry assay was applied to evaluate the percentage of necrotic, apoptotic, and normal cells. We selected the 2 ppm concentration of NPs for the flow cytometry test. For this purpose, after 24-h incubation of treated T. gondii tachyzoites with 2 ppm of NPs, the percentages of the apoptotic, necrotic, and normal cells were computed as 54.95%, 20.3%, and 24.3%, respectively. Nevertheless, the control group condition was calculated as 99.3%, 0.02%, and 0.70%, respectively. The results of flow cytometry analysis are depicted in Figure 3.
Flow cytometry results of the effect of silver nanoparticles based on ginger extract with the IC50 concentration on tachyzoite viability (B) and comparing with the control group (A). The right-bottom part shows early apoptosis; the right-top part shows secondary apoptosis; the left-top part shows necrosis; the left-bottom part shows living cells.
5. Discussion
Toxoplasma gondii infects about one-third of the world’s population (1, 2). Conventional medications for toxoplasmosis treatment have defects; they are ineffective on tissue cysts and difficult to tolerate by immunized patients. Also, sulfadiazine and pyrimethamine have several side effects, including increased platelet counts, neutropenia, leukopenia, thrombocytopenia, blood abnormalities, hypersensitivity reactions, etc. (24). Other medicines, including spiramycin, atovaquone, dapsone, and cotrimoxazole were also clinically tested, which showed weak effects on bradyzoites (25, 26). In previous research, NPs have been commonly used as vehicles for distributing drugs or vaccines and enhance their therapeutic efficacy (27). Although there are several studies on antibacterial, antiviral, and antifungal activities of Ag-NPs, no research has been published on the anti-parasitic effect of ginger-based Ag-NPs on T. gondii. Thus, our goal in this study was to assess the anti-parasitic effect of Ag-NPs on T. gondii based on the ginger extract.
In this study, we used different concentrations of Ag-NPs in the culture plate against T. gondii. It was found that the greater the NPs concentration and the longer the exposure period of tachyzoites, the greater the lethal effect on the parasite. Silver nanoparticles gave the best outcomes on the parasite alone or in combination with other native or nano-forms (28). For example, in agreement with our study, in a study in 2012, Said et al. (28) measured the anti-parasitic ability of nanoparticles of silver, chitosan, and curcumin as anti-Giardia agents. Silver nanoparticles were used for experimental giardiasis therapy and showed the best performance. Also, Allahverdiyev et al. (29) described the antileishmanial effect of Ag-NPs on Leishmania tropica by investigating their influence on different cellular parameters of promastigote and amastigote types. The researchers also boost the effect of Ag-NPs by presentation to UV rays, which increased their effects by 6.5 folds. They showed that the use of Ag-NPs may provide a potential alternative to current anti-leishmanial drugs. In addition to parasites, some research has suggested the high efficacy of Ag-NPs in inactivating bacteria and viruses. Cho et al. (30) examined the antibacterial impact of Ag-NPs. It has been indicated that Ag-NPs kill bacterial cells via various processes. In a study by Lara et al. (31) performed in 2009, the antibacterial activity of Ag-NPs was established against some drug-resistant bacteria, including Pseudomonas, Streptococcus, and Escherichia coli. The study found that Ag-NPs inhibited cell wall synthesis and some bacterial proteins by affecting the 30s unit of ribosomes in bacteria. There is also another study conducted by Kim et al. (32) in 2009, which examined the antifungal activity of three separate types of Ag-NPs against the unidentified ambrosia fungus Raffaelea sp., which caused the mortality of a large number of Korean oak trees. Examination with an electron microscope revealed that NPs could damage and rupture the fungal cell wall and prevented the growth of the fungus. The NPs also prevented the fungal conjunctival growth compared to the control group (32). In another study in 2010, the effect of Ag-NPs based on the leaves of the Mimosa pudica plant was evaluated on the larvae of malaria vectors, including Phlebotomus, Culex, etc. The maximum effect of hybrid NPs was observed on Rhipicephalus microplus, Culex quinquefasciatus, and Anopheles (33).
In our research, Ag-NPs based on ginger extract led to the induction of around 55% apoptosis in T. gondii. In a study in 2010, Gopinath et al. (34) investigated the effect of Ag-NPs on gene expression to identify the essential mechanisms leading to programmed cell death induced by silver NP. They employed RT-PCR to analyze the expression of genes, flow cytometry analyses to test apoptosis rates (FACS), and atomic force microscopy (AFM) to monitor differences in cell membrane topology induced by Ag NPs. According to this study, Ag-NPs activated the P53 gene and induced apoptosis in the target cell. It also made apoptotic changes to the cell membrane examined by electron microscopy (34). Another experiment conducted in 2011 identified the apoptotic effects of this type of NPs through fluorescence and RT-PCR techniques. The NPs could depolarize mitochondrial membranes and activate the caspase 3 enzyme. Due to this type of molecular activity, it is expected that this method will be used to treat cancer cells (35).
5.1. Conclusions
Based on the above results, it is concluded that silver nanoparticles based on ginger extract have a lethal effect on T. gondii and induce apoptosis in this parasite. This study encourages further studies in vivo.