1. Background
The annual incidence of death from Head and Neck Squamous Cell Carcinoma (HNSCC) is more than 380.000 cases (1). Regarding the stage of HNSCC, different treatment protocols are selected, each of which leads to different side effects (2). Photodynamic Therapy (PDT) is a treatment modality for precancerous lesions, with few adverse effects. Several studies reported the successful treatment of early-stage HNSCC with PDT (3). The PDT is approved by the US Food and Drug Administration (FDA) for the elimination and restriction of cancers, but research on the effectiveness, mechanism, and gene expression of malignant cells can lead to more effective treatment.
The basis of PDT is the activation of a photosensitizer that accumulates more and longer in cancer cells than in healthy cells. Radiating a wavelength of visible light with controlled irradiation time causes the production of Reactive Oxygen Species (ROS) and free radicals in cancer cells, which selectively destroy fast-growing cells (4). Photofrin was the first photosensitizer compound accepted for clinical use in PDT. However, due to several disadvantages, especially long-term sensitivity to light, the second and third generations of photosensitizers are being investigated (5). Studies have led to the development of other photosensitizer components, including aminolevulinic acid (ALA), methyl aminolevulinate (MAL), Verteporfin (BPD, benzoporphyrin derivative), Foscans (temporfin, meta-tetrahydroxyphenyl chlorine), methylene blue, and toluidine blue (6).
Cells use apoptosis as a mechanism to remove inefficient and harmful cells, which is regulated by several factors; one of these regulatory factors is P53 (7). P53 is a tumor suppressor gene. When an irrecoverable mutation occurs in the cell's genome, P53 eliminates the cells by activating the apoptosis pathway using Bcl-2 family proteins (8). The P53 encoding gene in cancers is repeatedly mutated and damaged as an oncogene, increasing the likelihood of uncontrolled cancer-like divisions in a benign cell (9). Patients with tumors whose P53 marker is mutated have a lower chance of survival. The P53 mutation and dysfunction are reported in more than 50% of human cancers (10).
Cyclin D1 is a 45 kDa protein coded by the CCND1 gene located on chromosome 11q13 (11). During the various stages of cell phases, the cyclin D1 (CCND1) is part of the molecular system that plays an important role in regulating the passage of the cell cycle from the G1 phase to the S phase (12). Therefore, after production, this protein forms complexes with CDK4 and CDK6 and causes the phosphorylation of retinoblastoma (RB) protein (13). With the phosphorylation of RB, the inhibition of EF2 transcription factor activity is removed, and the cell enters the S phase (14). Contrary to the normal function of CCND1 as a key protein and a positive regulator of the progression of the regular cell cycle, its overexpression causes the shortening of the G1 phase and the lower dependency of the cell on growth factors (15). These conditions can lead to the loss of natural control of the cell cycle and, subsequently, uncontrolled cell proliferation. It has been shown that increasing the CCND1 mRNA level may increase the growth potential and tumorigenicity (16). It has been reported that the mRNA relative levels change during PDT (17). PDT can reduce the severity of chemoradiotherapy‐induced oral mucositis and is associated with the reduction of inflammation (18).
2. Objectives
The aim of this study was to determine the effect of PDT using toluidine blue as a photosensitizer on the CCND1 and P53 mRNA levels in the HNSCC cell line.
3. Methods
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (IR.TUMS.DENTISTRY.REC.1396.2040).
3.1. Cell Culture
The human HNSCC cell line was prepared from the Pasteur Institute of Iran, Tehran, from the NCBI.C196 designation HN5 cell line. Cells were cultured in six-well plates in Dulbecco's Modified Eagle's Medium (DMEM) plus 10% sterilized FBS at 37°C with 5% CO2 and 1-2% moisture for at least 24 hours. After the completion of the culture, cells were divided into four groups: group I (HNSCC cell line under the influence of toluidine blue and laser irradiation), group II (HNSCC cell line under the influence of toluidine blue), group III (HNSCC cell line under laser irradiation), and group IV (control group, HNSCC cell line).
3.2. Cell Uptake
A 660 nm diode laser (THOR® Laser, London, UK), with a constant power of 200 mW, continuous mode, and energy density of 2.5 J/cm2, was used. The laser was calibrated using a power meter at 2.5 J/cm2 fluency because it was most effective among fluencies of 0.1, 0.2, and 1 J/cm2 over a wide range of toluidine blue concentrations in monolayers. Toluidine blue was examined at 1, 2, and 4×10-6 mol/L concentrations. Cell toxicity of toluidine blue at these concentrations was examined by the MTT assay. Based on the MTT assay, 4×10-6 mol/L concentration was suitable. The laser beam was irradiated to the cells perpendicularly in the non-contact mode. Regarding the area of the plates (24 cm2) and the spot size of the laser (1 cm2), the time of laser irradiation with sweeping movements was set at 20 minutes. Also, the photosensitizer substance of extra pure toluidine blue (25 g) prepared from Shimiran (Tehran, Iran) was used for the other part of the experiment.
3.3. Photoreaction Process
Two cell groups (I and II) were exposed to toluidine blue for three hours and then washed twice with normal saline.
3.4. RNA Isolation (RNA Extraction)
The RNA purification was performed manually using Trizol (RNX-PLUS; Cinnagen, Tehran, Iran). At least 106 cells were harvested, centrifuged, and washed with 2 ml of cold PBS (500 rpm, 3 min, 4°C). After washing cells for homogenization, 1 ml of Trizol (RNX-PLUS; Cinnagen, Tehran, Iran) was added to the samples, and the samples were stored at 25°C for 5 min. Then, 250 µl of chloroform was added. The samples were vortexed for 15 seconds and incubated for five minutes on ice, followed by centrifugation (12000 rpm, 15 min, 4°C). After centrifugation, three layers were observed; the transparent supernatant layer contained RNA, the thin and white middle layer contained DNA and protein, and the milky colored bottom layer contained other compounds. The transparent supernatant layer was slowly transferred to a new RNase-free microtube. To precipitate the RNA, cold isopropanol was added in a volume equal to the supernatant, followed by several times turning slowly upside down. They were incubated for 15 min on ice and then centrifuged (12000 rpm, 15 min, 4°C). The supernatant layer was removed, and to wash the pallet, 1 ml of 75% ethanol was poured over the precipitate, followed by gentle shaking (turning upside down) and centrifugation (7500 rpm, 8 min, 4°C). The supernatant was discarded, and the microtube was put in a sterile environment and real-time (RT) with the lid open to dry completely. After complete drying, the pellet was dissolved in 50 µl of diethylpyrocarbonate (DEPC) water.
3.5. Concentration and Optical Density (OD) Ratio
The concentration and purity of the extracted RNA were measured by a NanoDrop machine (IMPLEN, Germany). The RNA at the 260 nm to 280 nm optical density ratio (OD260/280 nm) ranged between 1.80 and 2.10, and the concentration of RNA was measured and set at 500 ng/µL.
3.6. cDNA Synthesis
The cDNA synthesis for real-time PCR was performed by the TaKaRa kit (TaKaRa, Japan) according to the kit instructions with the final volume reaction of 20 µl. The final reaction was synthesized in a thermocycler device for 15 min at 37°C and then 5 s at 85°C.
3.7. Primer Design for Real-time PCR
Specific primers of each marker were designed using AlleleID 7.0 software (Premier Biosoft International, California, USA) (Table 1). To increase the specificity of real-time PCR and the prevention of false-positive results due to the proliferation of DNA nucleotide sequences, the binding site of primer pairs related to specific genes was designed in one of the Exon-Exon junction regions. This led the primer to attached to sequences that existed in the cDNA derived from the mRNA.
| Gen | Sequence |
|---|---|
| P53-F | GATAGCGATGGTCTGG |
| P53-R | GTAGTTGTAGTGGATGGT |
| CCND1-F | CCCTCGGTGTCCTACT |
| CCND1-R | ACTTCTGTTCCTCGCA |
| HPRT-F | TGGACAGGACTGAACGTCTTG |
| HPRT-R | CCAGCAGGTCAGCAAAGAATTTA |
Primer Sequences of Markers
3.8. Real-time PCR
The real-time PCR was performed by Amplicon master mix containing SYBR Green fluorescence stain (Takara, Otsu, Shiga, Japan). Time and temperature profiles of the reaction were adjusted according to the kit instructions. After the end of each reaction, the interpretation of the results was done based on the amplification and melting peak curves (Table 2).
| Real-time Step | Temperature | Duration |
|---|---|---|
| Initial activation 40 cycles of: | 95°C | 150 seconds |
| Denaturation | 95°C | 15 seconds |
| Annealing | 60°C | 15 seconds |
| Extension | 72°C | 15 seconds |
Temperatures and Duration of Real-time PCR
In addition to the main samples containing cDNA (template), a sample with all components without cDNA as NTC (negative control) was set up. Negative results indicated that there was no contamination of materials used in the reaction. For the assurance of sample quality and quantity, positive control was used in two steps. In the first step, the optical absorption of the template was recorded, and in the second step, the mRNA relative levels of HPRT (Housekeeping gene) were evaluated. The template was diluted to control the quality of real-time PCR. For all P53, HPRT, and CCND1 mRNA, an appropriate dilution was considered as 1. To examine the mRNA relative levels, the fold change method was used. The Cycle Threshold (CT) values related to P53, CCND1 mRNA, and HPRT reference (Housekeeping gene) were determined in each of the samples. The P53 and CCND1 mRNA values were subtracted from the HPRT value. From the values obtained, the mean ΔCT (for each sample) was calculated and an average was determined for each group. These two values were subtracted from each other and eventually, the ΔΔCT was obtained. By raising 2 to the power –ΔΔCT (i.e., 2–ΔΔCT), the difference between CCND1 and P53 mRNA with HPRT was determined. This value represented the difference in the number of primary copies of P53 and CCND1 mRNA. The one-way ANOVA in SPSS (SPSS 22 for Windows; SPSS Inc., Chicago, USA) was used for analyzing the data.
4. Results
4.1. Examining the Purity and Concentration of Extracted Samples
The purity and concentration of extracted RNA and cDNA obtained from the reverse transcription reaction were measured by the method mentioned earlier (Table 3).
| RNA | cDNA | Wavelength (nm) | |
|---|---|---|---|
| Absorbance | 0.15 | 2.861 | 260 |
| 0.07 | 1.716 | 280 | |
| 1.9 | 1.667 | 260/280 | |
| Concentration (ng/μl) | 0.15 | 1.888 |
Purity and Concentration of Extracted RNA and cDNA Obtained From Reverse Transcription Reaction
4.2. Results of Real-Time PCR
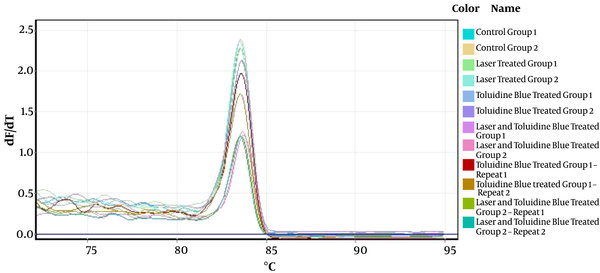
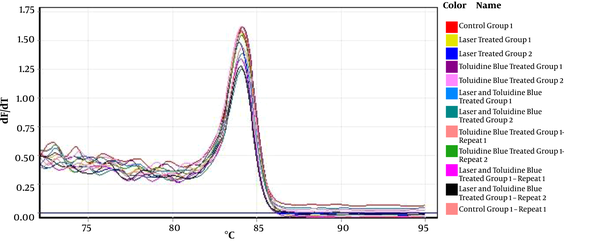
The interpretation of the results was made based on the melting peak curve using the fold change method. The melting curves of P53 and CCND1 in some samples are shown in Figure 1 and Figure 2, respectively.
The comparison of the fold change index for P53 mRNA in the study groups by the one-way ANOVA test showed that despite the increased expression of P53 mRNA in the treated groups compared to the control group, and there was no statistically significant difference in the increases in this index between the groups (P = 0.99). The mean increase in the P53 mRNA relative levels was 1.50 ± 0.33 in group I, 1.49 ± 0.23 in group II, and 1.40 ± 1.05 in group III. The comparison of the fold change index for CCND1 mRNA in the study groups using the one-way ANOVA test showed that there was no statistically significant difference in the increases in the CCND1 mRNA index between the groups (P = 0.22). Compared to the control group, the mean increase in CCND1 mRNA was 18.01 ± 3.37 in group I, 17.69 ± 3.3 in group II, and 9.01 ± 6.17 in group III (Table 4).
| mRNA | Mean | Standard Deviation | P Value in Comparison with the Control Group |
|---|---|---|---|
| P53 | |||
| Group I (laser + toluidine blue) | 1.50 | 0.33 | 0.65 |
| Group II (toluidine blue) | 1.49 | 0.23 | 0.50 |
| Group III (laser) | 1.40 | 1.05 | 0.63 |
| Control group | 1.00 | 0.00 | - |
| CCND1 | |||
| Group I (laser + toluidine blue) | 18.01 | 3.37 | 0.04 |
| Group II (toluidine blue) | 17.69 | 3.30 | 0.03 |
| Group III (laser) | 9.01 | 6.17 | 0.20 |
| Control group | 1.00 | 0.00 | - |
The Mean Levels of p53 and CCND1 mRNA in Control and Treated Groups
5. Discussion
Choosing the best treatment plan for HNSCC is a multilateral and critical decision due to the anatomical and functional characteristics of this area. The complications of each treatment modality for the local control of the tumor can affect the life quality of patients. In recent years, PDT has made remarkable progress in the treatment of primary cancers of the head and neck with minimal complications, and it can be used as an auxiliary treatment during surgery of advanced lesions (3).
The previous studies emphasized the positive effects of PDT on the control and treatment of cancers. Biel et al. studied the effects of photodynamic therapy (Photofrin; under the radiation of 400 nm, with an optical dose of 50-75 j/cm2) on the treatment of early stages of head and neck cancer. They reported that PDT was comparable to conventional treatments, while post-treatment complications were lower without causing significant impairment in the performance and aesthetic outcome of patients (19). Cooper et al. reported a complete recovery of 67% of squamous cell carcinoma lesions in the oral and oropharynx areas with the use of photodynamic therapy (mTHPC; laser diodes with a wavelength of 625 nm and a dose of 20 j/cm2) (20).
In this study, the effects of PDT with low-level laser were evaluated on the p53 and CCND1 mRNA levels in HNSCC cells. The results showed that despite the increased level of P53 mRNA in the treated groups compared to the control group, there was no statistically significant difference in this index between the groups. Also, the results of this study showed a significant increase in the CCND1 mRNA levels in the group treated with toluidine blue and the group treated with laser and toluidine blue compared to the control group. It is important to note that in the real-time PCR method, the fold change is used as a measure of gene expression. Based on this measure, after photodynamic treatment with toluidine blue, the p53 and CCND1 mRNA levels increased in comparison with the control group and other treatment groups.
Normal P53 mRNA cells with the mechanism of DNA damage can lead to cell apoptosis, but mutant P53 has oncogenic effects. The main function of P53 is to protect cells against genomic changes resulting from DNA damages. In human tumors, P53 is inactivated or has an oncogenic activity that comes from a point mutation in this gene (21). Lim et al. reported that PDT could induce an increase in P53 mRNA that may result in P53-related apoptosis. The results of the Lim study are compatible with our results that showed PDT could increase the P53 mRNA level and apoptosis. In the Lim study, the energy density of the laser was 6.25 j/cm2, whereas, in our study, it was 2.5 j/cm2. The increase of the P53 mRNA level in Lim’s study was statistically significant that may be attributed to the differences in study design and cell types. They used a combination of PDT and recombinant adenovirus P53 to induce cervical cancer cell apoptosis (22). Mitsunagas et al. study indicated that cell apoptosis in colon cancer depends on the P53 and BAX genes. They stated that PDT could increase the P53 mRNA relative levels. The results of this study were compatible with ours (23). Regarding the increases of P53 mRNA levels in treatment groups, it can be concluded that PDT with toluidine blue promoted cell apoptosis in the HNSCC cell line.
Also, the results of the current study showed a significant increase in the CCND1 mRNA level in the group treated with toluidine blue compared to the control group. The CCND1 gene located on chromosome 11q13 is a positive regulator of the cell cycle. It encodes the nuclear protein that forms complexes with cyclin-dependent kinases 4 and 6, phosphorylating and inactivating the retinoblastoma protein (pRb). The inactivation of pRb allows cell cycle progression from G1 to S phase. The CCND1 overexpression has a positive relationship with more advanced clinical stages of HNSCC (11, 24). The CCND1 overexpression was correlated with lymph node involvement, large tumor size, advanced clinical stage, and lower survival rate (25). The results of studies considering CCND1 as a marker of response to treatment are inconsistent. Zhong et al. stated that low-level expression of CCND1 mRNA predicted a worse prognosis in HNSCC, but the CCND1 mRNA levels did not predict response to treatment in this study (26). Shintani et al. found that HNSCC with the elevated CCND1 mRNA level was more sensitive to radiotherapy than were tumors with reduced expression of CCND1 mRNA (27).
Barberi-Heyob et al. indicated that increasing P53 expression can lead to cell photosensitivity enhancement. They suggested that the introduction of P53 in cancer cells potentiates the cell sensitivity to PDT through the induction of apoptosis (28). The results of their study may indicate that the increased expression of P53 mRNA level can induce cell apoptosis, which confirms the results of our study. The role of P53 in photo-induced cell death is controversial and depends on tissue type, and photosensitizer applied (29). P53 mRNA plays several important roles in different cellular processes (30).
PDT could be considered as an appropriate treatment for HNSCC. Due to aesthetic reasons and lower side effects, PDT is more tolerable for patients than are conventional cancer treatments. In the clinical view, post-treatment recovery time is shorter in this method than in other methods. Studies on the effects of PDT on cellular mechanisms and gene expression are limited. In this study, the effect of PDT was assessed on the expression of two mRNA levels involved in apoptosis and cell cycle progression. PDT with toluidine blue as a photosensitizer could increase the P53 mRNA level that is a key factor in promoting cell apoptosis. In our study, the group treated with laser and toluidine blue showed an increased CCND1 mRNA level that indicates the effects of toluidine blue on the apoptosis of HNSCC cells, but it is not the single marker of response to treatment. For a complete assessment of HNSCC cell lines’ response to PDT, the examination of cell apoptosis and necrosis and their cellular pathway is necessary. There are limited studies on the effects of PDT on gene expression in head and neck cancer. The results of this study could be a starting point for further studies in this area to provide more effective therapies for treatment-seekers in the future.