1. Background
Military police training should characterize the necessary abilities to conduct their activities with excellence. They all must be physically and psychologically prepared to guarantee their own safety, the safety of others, and also of society regarding the fact that these professionals frequently endanger their lives (1).
That said, the training needed to achieve this excellence is very arduous, requiring exhaustive simulations to prepare the trainees for the realities that await them in the field (2). Since special operations groups (SOGs) require unique operational capabilities, this issue is even more evident for them. As physical, biochemical, and psychological parameters heavily influence the performance of individuals, concerning both excellence in performing activities and longevity of the career, special attention should be paid to these parameters (2).
Battalions of Special Police Operations must deal with exceptional cases within the military police, acting in the fields of public defense, with a mission to solve problems that are beyond the aptitude of the operational units of the military police (3). Therefore, these groups should be made up of highly armed and specialized troops, working to combat heavily armed gangs on their missions in either urban or rural counter-guerrilla fields (3).
Therefore, laboratory analyses not only would provide valuable information for activities that cause great physiological stress (4), but also are useful for monitoring the training and nutritional status as well as identifying possible complications caused by the practice of a particular modality and/or work activity, consequently, focusing on longevity and health (4).
Within this line of reasoning, hormones are chemical signals secreted by specialized glands or cells, which help to control various functions in the body. Hormones stimulate signals to both distant cells (endocrine action) and adjacent tissues (paracrine action). These stimulations always act selectively, because only the cell that has a certain hormone receptor can respond to the stimulus (5).
Since a certain type of hormone can influence another (either an amplifying or suppressing effect), they act in a synchronized manner, not in isolation. Therefore, joint harmony is necessary to demonstrate good functioning (5). Regarding the action pathways of hormones, they can be used as important metabolic biomarkers of training and nutritional status.
Testosterone is a steroid male hormone that circulates in the body by binding to Sex Hormone Binding Globulin (SHBG), having only a small free-flowing portion. However, based on the free hormone theory, this small free portion binds to androgen receptors (6). Testosterone is responsible for several important functions in the body, including having a fundamental role in protein and lipid metabolism (7). In humans, this hormone has a great influence on body composition and muscle mass (7). Several symptoms are associated with testosterone deficiency, including increased central adiposity, reduced insulin sensitivity, increased triglycerides, and reduced HDL. These factors, in turn, contribute to metabolic syndrome and diabetes mellitus, as well as the increased risk of cardiovascular problems (8). Therefore, to perform an adequate evaluation, it is necessary to measure both total and free testosterone as well as its main binding protein. Dehydroepiandrosterone sulfate (SDHEA) is one of the most abundant steroid hormones in humans, which is produced by the adrenal glands. The SDHEA is a steroid hormone precursor (such as testosterone). Moreover, it also has an important role in the immune system, which justifies the necessity for its adequate levels of production for the proper functioning of the body (9).
Historically, it was believed that testosterone solely plays several key activities. Nevertheless, now it's well proved that several other hormones (e.g., estradiol) also contribute to functions that were historically attributed to testosterone, mainly because testosterone can undergo peripheral aromatization and transform into estradiol (about half of male estradiol have this origin) (10). The other portion of this hormone is produced from testicular secretion. In men, estradiol exerts important actions on bones, fat mass, and insulin resistance, which makes it an important hormone for analysis and one which is often overlooked in males (10).
When taking into account the importance of the balance of hormones and their binding proteins, presenting values within the normal range, according to age groups, are vital for health, contributing to the proper functioning of the metabolism of proteins, carbohydrates, and lipids.
If we believe that these hormonal values are of crucial importance for the normal population, when it comes to professionals who work in stressful conditions that need maximum efficiency of their organism, a higher value should be given to them. Concerning police officers working in SOGs, it should be noted that any disturbance can put their lives at unnecessary risk and may even cause them to leave work due to a health problem, a fact that can harm both the individual and society as a whole.
Therefore, hormonal monitoring is of great value since it provides a view of the current state of the subjects who are constantly exposed to extreme professional challenges.
2. Objectives
Within this line of reasoning, the present study aimed to evaluate the profile of steroid hormones and the sex hormone-binding protein of soldiers belonging to a special operations force.
3. Methods
3.1. Study Design and Sample Population
In this cross-sectional study, blood samples of 75 volunteers who were members of a SOG were collected after 12 hours of overnight fasting. Before collecting blood samples, the subjects were instructed to remain at rest and not perform vigorous activities before the analyses. The blood collection was performed at the battalion headquarters. Inclusion criteria were being an active member of a SOG, having medical clearance, and willingness to participate in the study.
3.2. Ethical Considerations
The present study is approved by the ethics and research committee of the Faculty of Physical Education and Sport of Ribeirão Preto EEFERP/USP (n. 3.230.057). Besides, written informed consent was obtained from all participants, and the researchers were available to answer any question regarding the objectives of the study.
3.3. Experimental Procedure
Blood samples were collected by puncture via access to the blood vessel through the anterior aspect of the forearm. Before collection, the forearm was properly cleaned with 70% ethanol. In total, 5 ml of blood were collected in tubes with separator gel to obtain the serum and immediately stored at 0 to 4ºC. Afterward, samples were centrifuged at 3000rpm in a temperature ranging from zero to 4ºC for 15 minutes to separate the serum. Then, serum was removed from the collection tube and stored in 1.5mL tubes for subsequent freezing at -80ºC. The remaining contents of the collection tubes were disposed of appropriately.
The analyses of total testosterone, free testosterone, SHBG, S-DHEA, and estradiol were performed with the chemiluminescence method, using blood serum, and were dosed by an authorized laboratory in the city of Rio de Janeiro - CNPJ: 07.727.439 / 0001 -60 - License: 1402.
Anthropometric assessment of weight and height was performed using a stadiometer with a 0.1 cm measurement scale and a Plenna digital scale model MEA-07400 with 100g accuracy, respectively.
3.4. Statistical Analysis
Data were analyzed using descriptive statistics (mean and standard deviation) and compared to the reference values for each biomarker, referencing each individual in their age range classification. SPSS version 22.0 was used for statistical analysis.
4. Results
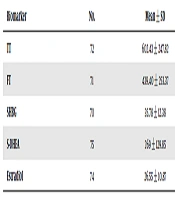
The mean age of participants was 37.23 ± 6.08 years. Also, their average weight and height were 80.4 ± 9.9 kg and 1.7 ± 0.05 m, respectively. An overall view of biochemical analyses of samples is provided in Table 1.
| Biomarker | No. | Mean ± SD | Min | Max |
|---|---|---|---|---|
| TT | 72 | 602.43 ± 247.82 | 117.82 | 1944.21 |
| FT | 71 | 439.40 ± 253.37 | 83.11 | 2312.51 |
| SHBG | 70 | 35.78 ± 12.38 | 15.8 | 73.4 |
| S-DHEA | 75 | 269 ± 129.85 | 57,55 | 768,1 |
| Estradiol | 74 | 26.55 ± 10.87 | 10 | 77,27 |
Abbreviation: TT, total testosterone; FT, free testosterone; SHGB, sex hormone-binding protein; S-DHEA, dehydroepiandrosterone sulfate.
4.1. Total Testosterone (TT)
Due to technical reasons, three samples were excluded from the analyses. Therefore, in total 72 blood samples were studied. The mean concentration of TT was 602.43 ± 247.82 ng/dl. As the classification of this hormone varies according to age, each individual received an individual classification. Since the youngest and oldest participants were 26 and 54 years old, the following classification was applied as reference values: 21-49 years (240.24 – 870.68 ng/dl); ≥ 50 years (220.91–715.81 ng/dl). Using this framework, only nine individuals presented values outside the normal range; among these, one was below the normal range, and eight were above the recommended threshold.
4.2. Free testosterone (FT)
For the FT values, that is, the testosterone that is not bound to any protein, the group mean was 439.40 ± 253.37. The mean was calculated considering 71 individuals since four samples did not obtain a reading. The reference value for this variable is unique, with no difference between ages. Thus, a parameter range of 131.00 - 640.00 pmol/L was considered to be ideal. The group value was within the recommended. When analyzing the individual results, only four individuals presented values outside the normal range; among these, three were above the ideal and one below.
4.3. Sex Hormone-Binding Protein (SHGB)
In this part, five samples were removed due to not obtaining a reading. Hence, in total, 70 samples were analyzed. The mean SHGB of the whole group was 35.78 ± 12.38. The reference value is unique for this protein, with a normal range of 11.20-78.10 nmol/L. The overall mean was in the normal range. Furthermore, for individual participants, also the obtained values were in the normal range.
4.4. Dehydroepiandrosterone Sulfate (S-DHEA)
For the S-DHEA, the overall mean for all 75 samples was 269.00 ± 129.85. The normal value defined for the S-DHEA varies by age. In this sense, the following classification was used as reference values: 25–34 years (167.90–591.90 ug/dl); 35–44 years (139.70–484.40 ug/dl); and 45–54 years (136.20–447.60 ug/dl). In this case, 17 individuals presented abnormal values, who 13 were below the recommended range, and only four were higher.
4.5. Estradiol
One sample was excluded due to not obtaining a valid reading. Hence, 74 samples were analyzed. The overall mean was 26.55±10.87 pg/ml. A single reference value is defined for the estradiol, covering all age groups, ranging from 11.00 to 44.00 pg/ml. The overall mean of the group fell in this range. Nevertheless, concerning individual samples, seven were out of the expected range (3 below and 4 above the recommended range).
5. Discussion
Older adults often are not efficient for performing activities that require high physical performance during working hours, mainly due to biological alterations caused by increased age, which led to declined functional capacity (11). The stability of hormonal factors indicates the biological preparedness of individuals, which is a very important factor for their training and overall performance (4).
In the present study, participants were quite diverse concerning their age. So that out of the total 75 participants, 27 were in the age group of 26-34 years, 23 in 35-39 years, and 25 in 40-52 years. Nevertheless, age was not a confounding variable in the analyses since, when refining the results, it was found that the older participants and their younger counterparts were well-classified, which is most likely the result of an extremely rigorous selection applied in the present study.
Testosterone has a fundamental effect on the body. So that several population-based studies have reported that testosterone deficiency significantly increases the mortality from any cause and is also an independent risk factor for cardiovascular diseases (12-14). It also causes several other negative health consequences as follows: impaired insulin sensitivity, increased body fat (mainly central fat), dyslipidemia, and hypertension (8). Therefore, based on the laboratory results, most of the participants of the present study are immune against negative health consequences of testosterone deficiency.
Overall, estradiol values were almost entirely within the normal range; however, a thorough analysis pointed to two individuals who presented very higher estradiol values. These subjects also had high values of TT and FT, which demonstrates that the excess of testosterone is aromatized to estradiol in the periphery through the aromatase enzyme, which belongs to the cytochrome p450 enzymes family (15). Although they were isolated cases, but their blood samples were analyzed by a multidisciplinary team, which performed the entire procedure for necessary corrections and adjustments.
The good concentration of S-DHEA found in the present study guarantees an adequate contribution to the supply of testosterone concentrations because although testicles are the main producer of testosterone, S-DHEA also produces this hormone (9).
Regarding the SHBG, a study has demonstrated that during intense military training, individuals who attained an overreaching state (excessive accumulation of stress that causes a momentary decline in performance) already had higher baseline SHBG values relative to before training. The authors argued that the enhanced concentration of testosterone results in its increased binding to the SHBG, which in turn decreases its availability, which may negatively influence the body responses during training (16). This issue highlights the importance of adequate levels of this binding protein.
Another study has shown that dietary restriction plus intense military training could increase SHBG and decrease the testosterone concentration, which in turn greatly reduced the availability of this hormone (17).
Continuous monitoring of hormonal concentrations and proteins that carry them is necessary to maintain the performance of highly active populations, as these analyses can provide valuable information regarding biomarkers that reveal biological changes of the body. This information can also be used to change behaviors to prevent negative consequences (18).
Therefore, when performing hormonal analyses, it is suggested to have a holistic approach, as investigating a sole hormone is unlikely to provide reliable information. Besides, applying a set of analyses creates an environment that favors a more reliable view of what is happening in the organism, which minimizes the possible misinterpretations that would have been occurred if an isolated hormonal fraction was followed.
In conclusion, it can be concluded that most of the elite soldiers investigated in the current study presented recommended values of steroid hormones and sex hormone-binding protein. Furthermore, including individuals of various ages didn’t change the good results found in the group.
Attributing positive results found in the present study in such a heterogeneous group with a diverse age structure to the extremely rigorous selection process and the training program that has elevated them to the rank of elite soldiers of the military police seems to be reasonable.