1. Background
It has been reported that oxidative stress from exercise may contribute to muscle damage and the development of overtraining symptoms such as fatigue and performance reduction in athletes (1). Decreased blood and tissue levels of vitamins and antioxidants that enter the body through diet, along with increased oxidative stress, expose target cells and tissues to oxidative damage caused by exogenous and endogenous oxidants (2). The body responds to oxidative stress by activating the defenses of antioxidants and other protective systems (2). Total antioxidant capacity (TAC) reflects the antioxidant properties of a range of antioxidant compounds in plasma (3). Also, ROS is a common mediator of the signal transduction pathway (4) and can stimulate the production of cytokines by multiple cells (4). For this reason, exercise induces cascading secretion of cytokines (5, 6). Interleukin-17 (IL-17) is a proinflammatory cytokine involved in regulating immune and inflammatory responses. Although the production of pre-inflammatory cytokines, such as IL-6 and IL-17, are essential for immune defense, overproduction of these cytokines may lead to inflammation and subsequent skeletal muscle damage, weakness, and increased risk of infection (7). Some researchers believe that plasma levels of IL-17 may be a useful biochemical indicator to determine acute inflammation produced in the skeletal muscle of humans or animals (8). It has also been reported that cytokine IL-17 is involved in the process of skeletal muscle inflammation (9) and the effect of exercise intensity on IL-17 has been considered as a determining factor in some studies (9, 10). Regarding the threat of oxidizing elements and the supportive effect of antioxidant variables on body function in terms of physical activity and sports, today, the emphasis is on the use of antioxidant supplements, such as vitamins E, C, carotenes, etc. Some researchers also believe that the use of nutrients or natural supplements in the daily diet can prevent the decline in performance due to the reduced power of the antioxidant defense system (11). There are very few studies on the role of natural supplements and foods containing polyphenols, flavonoids, isoflavonoids, and other antioxidants (12, 13). One of these plants is ginger (14). The beneficial effects of ginger on the body include antioxidant effects and scavenging of free radicals (15). Regarding the anti-inflammatory effects of this plant, it has been reported that the active ingredients of ginger, such as gingerol, shogol, and curcumin, have important physiological and pharmacological activities, such as antioxidants, anti-inflammatory, analgesic, and anti-cancer, and have a good ability to inhibit prostaglandins and interleukins, which are involved in inflammation (16)
2. Objectives
Due to the lack of information about the effect of ginger along with endurance training on cytokines and regarding the relationship between reactive oxygen species and cytokine production and despite the widespread interest in replacing natural supplements, especially in relation to physical and sports activities, the present study aims to investigate the effect of ginger extract supplementation and endurance training on plasma Interleukin-17 concentration and total antioxidant capacity of skeletal muscle tissue in male rats.
3. Methods
The study protocol was registered at Iran’s National Committee for Ethics Biomedical Research (IR.SSRC.REC.1396.143). The research was conducted experimentally. In the present study, 40 male Wistar rats (aged 8 weeks and average weight of 20 ± 200 g) were purchased from the Razi Serum Institute of Karaj. After being transferred from the breeding center to the research environment, the animals were kept in new conditions for 2 weeks. In the second week, all animals were introduced to how to work on the treadmill. Mice were randomly divided into 5 groups based on weight. However, during the study, due to the loss of some rats, the final analysis was performed on the remaining rats [control group (8 heads), sham (7 heads), endurance training (7 heads), ginger (8 heads), and endurance training + ginger (7 heads)]. All animals were kept at 22°C and light-dark cycle for 12:12 hours and 50% humidity. The food was produced by Pars Livestock Feeding Company, which was provided for free. The special ratchet was made in Iran and had a hand shocker and speed, time, and distance screens.
3.1. Training Protocols
The whole training period was divided into two stages:
(1) First stage (introduction stage): The acquaintance program consisted of 5 sessions per week for a week, which was performed by walking and running at a speed of 5 to 8 meters per minute (zero degree slope and duration of 5 to 10 minutes).
(2) The second stage (main exercise): It contained training programs with a speed of 10 m/min for 30 minutes in the first week, with a gradual increase (every week) to a speed of 35 m/min for 70 minutes (equal to 80 - 85% of the maximum oxygen consumption) during the last week (17). The training time was fixed for the training group, and the sports protocol was performed on Saturdays, Sundays, Mondays, Wednesdays, and Thursdays from 3 pm to 5 pm.
3.2. Method of Collecting the Plant and Preparing Ginger Sap
To prepare ginger sap, the plant’s rhizome was purchased from the market during the growing season and dried at home. One gram of pure alcohol was then added to one gram of ginger powder and diluted with normal saline to a volume of 00 cc (18). Mice were weighed before each session. Ginger sap was injected intraperitoneally at the rate of 00 mg per kg of body weight for 3 days a week (Saturdays, Mondays, Wednesdays) to the endurance-ginger group.
3.3. Tissue Sampling and Sampling Methods
The animals were anesthetized at the end of the training period and 48 hours after the last training session. The blood of rats was then collected in Falcon tubes and placed in a centrifuge (0 minutes at 3000 rpm), and the serum was isolated. The isolated horseshoe muscle tissue was placed in microtube tubes and stored in the freezer at -80°C for further measurement. Serum IL-17 index was measured using an ELISA kit made by BT (Bioassay Technology) of China. The sensitivity and coefficient of variation within the test were 0.92 pg/mL and 0.02%, respectively. Also, the TAC of the muscle was measured using the FRAP method by a kit made by the Zelbio company (Germany). The coefficient of variation within the test was 3.4%. FRAP is based on reducing ferric ions to Ferro by reducing the power of antioxidant compounds in each sample, which is measured spectrophotometrically at a wavelength of 593 nm. Statistical calculations were performed by SPSS software version 23. The Shapiro Wilk test was used to examine the normality of the data distribution. Due to the normal distribution of data, one-way analysis of variance and Scheffe post hoc test were used to compare variables in a research group. Statistical significance was considered when P-value < 0.05.
4. Results
4.1. Total Muscle Antioxidant Capacity
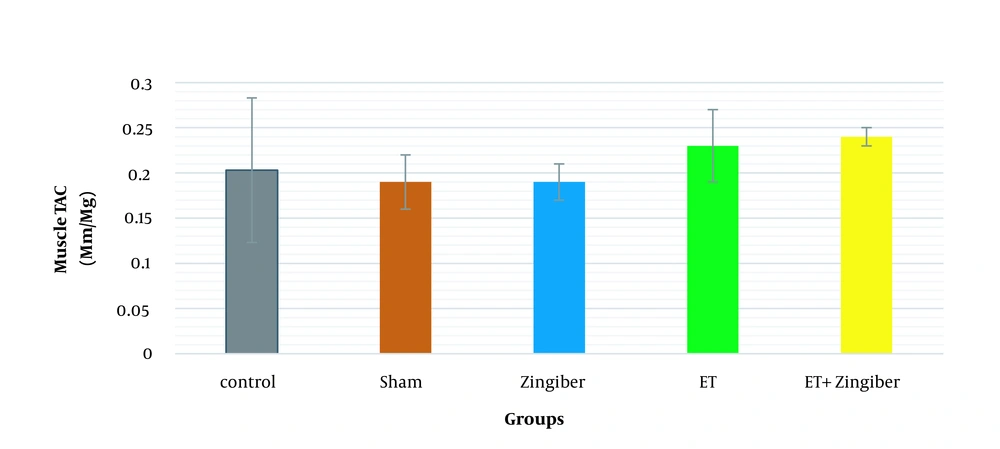
Analysis of data related to TAC of skeletal muscle tissue did not show a significant difference between the tested groups (P = 0.22, η2 = 1.50, F4.32 = 15.56). However, the TAC level of the training and exercise + ginger group had a non-significant increase of 13.04% compared to the control group (Figure 1 and Table 1).
Changes in total antioxidant capacity of muscle in the studied groups after 8 weeks; TAC, total antioxidant capacity; control, control group, Sham: Sham; ET, endurance training; ginger: ginger, ET + ginger: endurance training + ginger. Values are means ± SD. * Statistically significant (P < 0.05).
| Variables and Groups | Sum of Squares | df | Mean Square | F4.32 | P Value | Effect Size(η2) |
|---|---|---|---|---|---|---|
| Serum IL-17 (PG/MI) | 0.508 | 0.00 a | 0.5 | |||
| Between groups | 0.04 | 4 | 0.004 | |||
| Within groups | 0.076 | 32 | 0.002 | |||
| Total | 0.090 | 36 | ||||
| Muscle TAC (mm/mg) | 5.560 | 0.22 | 0.66 | |||
| Between groups | 359.35 | 4 | 8779.83 | |||
| Within groups | 8056.32 | 32 | 564.26 | |||
| Total | 5375.676 | 36 |
a A significant difference compared to the control group (P < 0.05).
4.2. Serum Interleukin-17
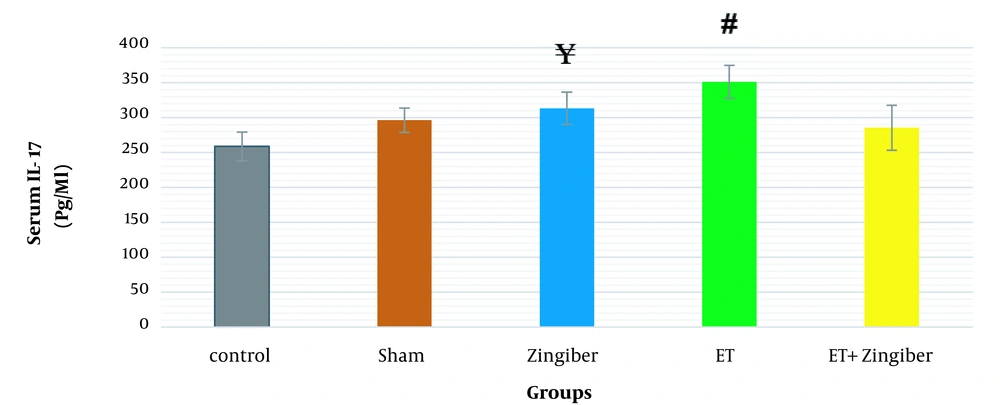
Analyzing data on the serum IL-17 showed a significant difference between the test groups (P = 0.00, η2 = 0.66, F4.32 = 15.56). The ETA square indicates how strong the effect of the group types is (Table 1). Also, the Scheffe post hoc test showed a significant increase (P = 0.00) in the level of serum IL-17 in the endurance group compared to the controls (P = 0.00) and sham (P = 0.004). In addition, serum levels of IL-17 were significantly decreased in the endurance + ginger group in comparison to the endurance group (P = 0.00). Also, the post hoc test showed that the serum level of IL-17 was significantly increased in the ginger group compared to the control group (P = 0.02) (Figure 2 and Table 2). The results are given in Table 2 in full.
| Group (I) and Group (J) | Mean Difference (I-J) | Std. Error | P Value |
|---|---|---|---|
| Control | |||
| Sham | -37.66 | 2.29 | 0.07 |
| Zingiber | -54.75 b | .87 | 0.002 |
| ET | -92.66 b | 2.29 | 0.00 |
| ET + Zingiber | -26.80 | 2.29 | 0.33 |
| Sham | |||
| Zingiber | -7.08 | 2.29 | 0.74 |
| ET | -55.00 b | 2.69 | 0.004 |
| ET + Zingiber | 0.85 | 2.69 | 0.94 |
| Zingiber | |||
| ET | -37.9 | 2.29 | 0.07 |
| ET + Zingiber | 27.94 | 2.29 | 0.29 |
| ET | |||
| ET + Zingiber | 65.85 a | 2.69 | 0.00 |
a The Dependent Variable is Serum IL-17 (PG/MI).
b A significant difference compared to the control group (P < 0.05).
5. Discussion
Overall, this study demonstrated that ginger supplementation, along with endurance training, caused a significant decrease in IL-17 and a non-significant increase in the TAC. So far, no study has investigated the effect of ginger on IL-17 levels. Also, there are limited and contradictory data on changes in the plasma and serum levels of IL-17 in humans. For example, Alizadeh et al. showed that after 8 weeks of exercise and completion of the research process (i.e., increase in speed, slope, and training duration), IL-17 levels were significantly increased in the experimental group (10). The results reported by Alizadeh et al. are consistent with the present study in terms of a significant increase in IL-17 in the training group compared to the control group. Duzova et al. also reported that IL-17 production increased in the long-term and after intense (2 weeks) activity. They reported no increase in the group that exercised for less time (weeks) and had moderate intensity (9). To explain the observed increase, they argued that intense exercise causes the release of some proinflammatory cytokines, which in turn produce anti-inflammatory cytokines such as interleukin-2 and interleukin-0. The subsequent production of these pre-inflammatory and anti-inflammatory cytokines are the reasons for the production of IL-17 by peripheral blood leukocytes and skeletal muscle (9). On the other hand, Golzari et al. found that using a combined program (including warm-up, stretching, strength training, and aerobic exercise) for eight weeks not only didn’t increase the level of IL-17 but also it was decreased in some cases (19). They attributed the decrease in IL-17 to low levels of exercise intensity. The discrepancy between the results of Golzari et al. and the present study is probably due to the difference in the training intensity of these two studies. Lowder et al. also studied the effect of 4 weeks of exercise on the treadmill and reported a decreased level of IL-17. They mentioned low exercise intensity as the main reason for the observed decrease (20). Therefore, according to the results of various studies, it can be argued that the intensity or duration of exercise is an important factor in increasing the production of IL-17. At the end of this discussion, it should be noted that although pre-inflammatory cytokines are essential for immune defense, overproduction of these cytokines may lead to inflammation and subsequent skeletal muscle damage, weakness, and increased risk of infection (21); because cytokine IL-17 can activate macrophages, fibroblasts, cytokine secretion of IL-, IL-6, and IL-8, prostaglandin E2 secretion, and nitric oxide (22). Thus, IL-17 induces and enhances inflammation by inducing the production of these factors and may lead to inflammation and subsequent skeletal muscle damage, weakness, and exacerbation of infection (7).
Another important finding of this study is the non-significant increase in TAC of skeletal muscle tissue in the exercise-ginger group and exercise group compared to the control group. In the study by Carvalho et al., after 8 months of training, a significant increase in TAC (protein 0%, uric acid 58% and ascorbic acid 27.03%) was observed. Since uric acid has a large share of this complex, enhanced plasma uric acid can be mentioned as a reason for increased antioxidant capacity after exercise (23). In a study by Jamurtas et al., intended to examine the effect of three different exercise programs (i.e., resistance training, endurance training, and combination training) on the total plasma antioxidant capacity in men, it was reported that TAC increased significantly in all training groups compared to the control group. One of the possible reasons for the observed increase was the implementation of long-term exercise, which may lead to the simultaneous adaptation of the antioxidant system in the body (24). Studies also show that consuming ginger may increase the body’s antioxidant capacity by increasing the activity of antioxidant enzymes in the blood, thus eliminating free radicals and reducing the body’s oxidative stress. In this regard, a group of researchers stated that 6-week supplementation with ginger, along with exercise, increased the antioxidant system, leading to reduced oxidative stress in obese women with a diagnosis of breast cancer (25). Possibly an increase in the total skeletal muscle antioxidant capacity following ginger supplementation in combination with endurance training may justify lower levels of IL-17 in this group. However, one of the limitations of the present study is not considering the response of different body tissues (skeletal muscle, heart, kidney, brain, etc.) to the independent variable and also changes in hormones such as catecholamines and cortisol during exercise, which is definitely helpful in scientific justification of the results. Therefore, it is suggested that researchers in future research control these issues to provide more reliable results.
5.1. Conclusions
According to the findings of the present study, 8 weeks of endurance training combined with ginger supplementation significantly reduced the inflammatory index of Interleukin-17 and strengthened the complete antioxidant system of skeletal muscle tissue. It can be argued that ginger, with fewer side effects than nonsteroidal anti-inflammatory drugs, is an effective supplement in reducing inflammation and improving the antioxidant system and reducing the damage in athletes.