1. Background
Low mobility can lead to chronic diseases such as diabetes, high blood pressure, cardiovascular disease (CVD), and obesity. Stagnant habits can reduce life expectancy. As part of a person's daily routine, regular exercise may help manage pathological conditions (1). Studies show a definite relationship between osteoarthritis (OA) and stillbirth due to inactivity, mainly due to diabetes and CVD (2). OA is an advanced degenerative process resulting in irreversible articular cartilage loss. It is characterized by pain and joint dysfunction. OA is considered the most common joint disease and one of the most common chronic diseases (3). CVD, prevalent in society, is the most common cause of death in developed countries (4). The incidence rate of OA and CVD increases with age, and they are among the most common diseases (3). Some studies show a strong relationship between OA and CVD (5). New meta-analyses show that OA is a serious risk factor for CVD (6).
In response to an injury, the Wnt/β-catenin signaling pathway, which is inactive in many adult organs, will be activated (7). The role of the signaling pathway in healing and regenerating incomplete and complex tissue is well understood. Moreover, growing data show that its activation increases fibrotic healing (8). Recent studies have shown that a short-term inhibition of Wnt therapy after cardiac ischemic injury improves the recovery with less fibrosis (9). In the pathogenesis of OA, due to increments in the expression of Wnt ligands and target genes, the focal Wnt signaling activation is observed in both synovial and postoperative cartilage (10).
In a canonical Wnt, the signaling pathway involves the β-catenin multifunctional protein. The β-catenin is usually known for its functionality in cell adhesion due to its interaction with the membrane-bearing proteins of the cadherin family. However, here, they act as a signaling mediator. In the absence of Wnt, the β-catenin cytosol interacts with destructive complexes such as Axin, APC, and GSK3β. Then, it is phosphorylated and subsequently degraded by protease. However, in the presence of a Wnt ligand, the destructive complexes are isolated. Then, the β-catenin is stabilized and enters into the nucleus to regulate the transcription of target genes by interacting with TCF/LEF transcription factors (11). Zhao et al. reported that blocking the Wnt/β-catenin signaling may be a new strategy to improve heart disease and high blood pressure (12). As demonstrated by Lietman et al. the inhibition of Wnt/β-catenin signaling improves OA in the experimental model of OA (13).
Today, physical activity is widely recommended by health care professionals in the prevention and management of chronic health conditions including cardiovascular disease, mental illness, and obesity. However, the effect of activity on the development or progression of osteoarthritis is contradictory. Some studies have shown that physical activity impacts osteoarthritis or joint health. It has been shown that walking activity has protective effects on the loss of joint space. Older adults who perform a high level of strenuous physical activity have an increased risk of radiographic knee osteoarthritis (14). An exercise-based and non-pharmacological treatment significantly benefits the lumbar environment, hypertension, and triglycerides (15). A meta-analysis of 33 studies showed that people who performed 150 minutes of moderate-intensity physical activity per week had a lower risk (14%) of developing CVD. Moreover, those with 300 minutes of moderate-intensity physical activity per week were 20% less likely to develop CVD (16). Even light to moderate activity (at least one hour of walking per week) is associated with lower rates of CVD (15).
Regular physical activity is a lifestyle intervention to improve all involved risk factors in CVD and blood glycemic level fall, which helps lose weight and increases insulin sensitivity in people with insulin resistance (17).
Some evidence suggests that exercise and mechanical stress may increase the Wnt pathway activity (18-21). Fujimaki et al. showed that chronic running led to the activation of satellite cells in diabetic mice and improved the Wnt signaling pathway (22). Pourrazi and Asgharpour Arshad showed that regular exercise improved the process of muscle mass loss due to dietary restriction by influencing Wnt signaling (23). However, there are very few studies on the effect of aerobic and endurance training on the Wnt signaling pathway and its proteins (such as β-catenin and glycogen synthase kinase-3) with conflicting results. Fujimaki et al. showed that four weeks of voluntary and intense running on a rotating wheel caused an over-adjusted Wnt route, resulting in the increment of β-catenin in the exercise group. The route activated satellite cells and then increased the myogenic genes copying in the exercise group (24). However, Amin et al. detected no change in the expression of β-catenin protein following exercise in rats (25).
HA is a polymer and a major component of the extracellular matrix in connective tissue. Moreover, it is a polysaccharide and an extracellular matrix glycosaminoglycan. HA has many interesting properties such as the capability of binding to water molecules. It is responsible for storing growth factors, binding to receptors, vascularization, proliferation, cell migration, and repairing damaged areas (26). The intra-articular injection capability of HA is one of the most common treatments for osteoarthritis, especially when other treatments are useless due to toxicity or ineffectiveness. Numerous studies have demonstrated the beneficial effects of intra-articular injection of HA on pain control and patient activity (26).
Mesenchymal stem cells (MSCs) are considered adult stem cells. They are known for their long-term renewal potential and ability to differentiate into skeletal cells. These cells are a type of non-hematopoietic cell living in the bone marrow and have been isolated and described for the first time. In adults, other sources of MSCs are periosteal tissue, trabecular bone, adipose tissue, synovial membrane, skeletal muscle, peripheral blood, and deciduous teeth. The differentiation capability of MSCs and their ability to reproduce over a long period have made them a viable source in tissue repair, cell therapy, and tissue engineering strategies (27).
In brief, the three methods of HA, exercise training, and MSCs are highly useful. However, previous studies have assessed them individually and never compared the three methods extensively. Hence, we conducted this study by comparing the effects of the three therapies on the Wnt/β-catenin signaling pathway in rats' heart tissue with an experimental model of knee OA.
2. Methods
The study was approved by the Animal Care and Use Committee at the Islamic Azad University of Sari (approval reference number: NO.19.33.2018).
2.1. Population and Statistical Sample
The statistical samples were 63 male Wistar rats with a mean weight of 250 - 300 g. The animals were divided into nine groups (seven in each group): (1) Healthy control, (2) patient control, (3) sham, (4) saline, (5) EXT, (6) MSCs, (7) HA, (8) EXT + MSCs, and (9) EXT + hyaluronic acid (HA).
2.2. Research Environment and Nutrition Subjects
After transferring the animals into the laboratory, they were housed in transparent polycarbonate cages measuring 15 × 15 × 30 cm (the Razi Rad Company). As an environmental condition, the researcher provided the ambient temperature of 20 ± 2°C, the humidity of 50 ± 5%, and proper ventilation. The required food was supplied by the pellet feed of the Karaj Animal Feed Company in a 500 mL bottle for the laboratory animals.
2.3. Osteoarthritis
For the OA induction, the animals were anesthetized with ketamine and xylazine. Then, a vertical incision was made on the pre-shaved and inner part of the right knee. After removing the skin, the inner lateral ligament of the knee was removed to allow for an internal meniscus. Next, an incision was made, which incompletely resulted in rupture and injury to the meniscus. The OA model was induced and, again, sutured using the sterile method. While performing all procedures, the minimum pain for the animal and the principles of working with laboratory animals were regarded. After the induction, a recovery period of three weeks was considered.
2.4. Training Protocol
The whole training course consisted of two phases:
- The first phase (familiarization): The purpose of this phase was to familiarize with the research environment and the tape drive. The rats were operated on a treadmill for four days, every day for 5 to 10 minutes at a speed of 6 to 8 m/min and a slope of zero percent.
- The second phase (main training): The training program consisted of 25 - 29 minutes of running on a treadmill with no slope at the speed of 15 m/min for the first week. The running duration was increased to 34 - 44 minutes progressively in the following weeks. The intensity reached 16 - 18 m/min in the fourth week (Table 1). The animals were warmed and cooled for five minutes before and after the training, respectively.
| Sessions | Exercise Factors | First Week | Second Week | Third Week | Forth Week |
|---|---|---|---|---|---|
| First | Speed (m/min) | 15 | 16 | 17 | 18 |
| Duration (min) | 25 | 30 | 35 | 40 | |
| Second | Speed (m/min) | 15 | 16 | 17 | 18 |
| Duration (min) | 26 | 31 | 36 | 41 | |
| Third | Speed (m/min) | 15 | 16 | 17 | 18 |
| Duration (min) | 27 | 32 | 37 | 42 | |
| Forth | Speed (m/min) | 15 | 16 | 17 | 18 |
| Duration (min) | 28 | 33 | 38 | 43 | |
| Fifth | Speed (m/min) | 15 | 16 | 17 | 18 |
| Duration (min) | 29 | 34 | 39 | 44 |
2.5. Hyaluronic Acid
Two successive intra-articular injections were separately administered to the HA receiving groups in a week. The intra-articular injections of 25 µL of HA (Hyalgan® sodium hyaluronate; Fidia Farmaceutici S.p.A., AbanoTerme, Italy) were administered using a 27-gauge/0.5-inch needle (28).
2.6. Mesenchymal Stem Cells
The bone marrow cells were collected from the femur and tibia. After culturing in the laboratory, each rat was injected with 1,000,000 cells per kilogram at the site of cell model induction (28).
2.7. Sampling
All the animals were sampled in similar conditions. The sampling was done after 12 to 14 hours of fasting and 72 hours following the last training session to eliminate the acute effects of exercise. The rats were anesthetized by the intraperitoneal injection as a combination of ketamine (70 mg/kg) and xylazine (3 - 5 mg/kg). According to the predetermined timing, cardiac tissue sampling was performed on the control and exercise groups. The samples were then washed with saline in special tubes and frozen in liquid nitrogen.
RT-PCR was used to analyze the expression of the β-catenin, GSK-3, Wnt, Fz, DKK1, and TCF genes in cardiac tissue. First, the tissue samples were homogenized in phosphate buffer (pH 7.0) at 4°C with a homogenizer. Total RNA was extracted from the samples using the RNX-Plus kit (SinaClon; RN7713C). A nanodrop ND-1000 spectrophotometer (Thermo Sci., Newington, NH) was used to estimate the quantity and quality of the extracted RNA. The synthesis of cDNA was done using the RevertAid Reverse Transcriptase (Thermo science, Germany) at 42°C for 1 hour (Thermo Science, Germany). For the amplification, a Rotor-Gene 6000 (Corbett Research, Australia) thermocycler and Real Q-PCR 29 Master Mix Kit (Amplicon, Denmark) were applied in 40 cycles. The mRNA level was normalized relative to the amount of GAPDH mRNA. The primer sequences were as follows (Table 2):
| Gene | Reverse Primer 5’ - 3’ | Forward Primer 5’ - 3’ |
|---|---|---|
| Gsk3b | TCCACCAACTGATCCACACCAC | CAAAGCAGCTGGTCCGAGG |
| Wnt3a | AGGAGACGGTTTCAGGGTTG | CGACTCAGAGATGGTGGTAGA |
| Ctnnb1 | ATGAAACTGCGTGGATGGGA | ATGCTGAGGAAGAAGATGTGGA |
| Tcf7l2 | CACCTTCCCTCTCATCTCCTTC | CATAGTCCCTCCCCATCACAC |
| Fz | TGATGGTGCGGATGCGGAAGAG | GAGTGGAGTGTGTTTTGTGGG |
| Dkk | GTAAATGGCTGTGGTCAGAGGG | TTGCGCGGAGGATGAGGAGTG |
| GAPDH | ATGGTATTGGAGAGAAGGGAGGG | GGATAGTGAGAGCAAGAGAGAGG |
2.8. Data Analysis Method
We utilized descriptive statistics to classify the data of this study. We applied the Shapiro-Wilk test to ascertain the normality of the data distribution. We practiced the one-way analysis of the variance to discover the significance of the differences between the variables and their interaction. We performed Tukey's post hoc test to determine the significance of the data. The results were analyzed at a 95% confidence level (P ≤ 0.05), and the IBM SPSS (version 20) was applied for the statistical analysis. All data were presented as mean ± SD.
3. Results
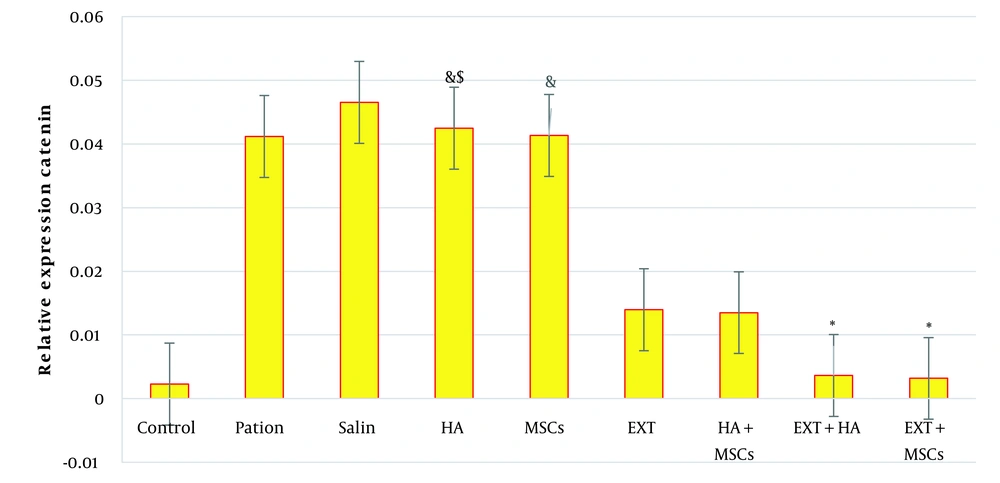
The mean and deviation criteria related to β-catenin gene expression in cardiac tissue in different studied groups are shown in Figure 1. Significant differences in the mean and standard deviation of β-catenin gene expression in cardiac tissue were observed between different groups (P < 0.000).
The results of the ANOVA test, F value (5/531), and P-value = 0.000 indicated a significant difference in β-catenin gene expression levels between different research groups. Based on these findings, the mean β-catenin gene expression in cardiac tissue in osteoarthritis mice increased significantly in the patient, saline, HA, and cell groups compared to the other groups. The other groups showed a significant decrease compared to the control. Accordingly, the EXT + MSCs to patient (92.25%), EXT + HA to patient (91.1%), EXT + MSCs to MSCs (92.28%), EXT + MSCs to HA (92.49%), and EXT + HA groups had a significant decrease compared to HA (91.44%) (Figure 1).
The mean and deviation criteria related to GSK3 gene expression in cardiac tissue in different studied groups are shown in Figure 2. Significant differences were observed in the mean and standard deviation of GSK3 gene expression in cardiac tissue between different groups (P < 0.000).
The results of the ANOVA test, F value (5.489), and P-value = 0.000 indicated a significant difference in GSK3 gene expression in cardiac tissue between different groups. Based on these findings, the average GSK3 gene expression in cardiac tissue in osteoarthritis mice was significantly reduced in the patient, saline, and HA groups compared to the other groups. The other groups showed a significant increase compared to the control group. Accordingly, the EXT + MSCs to patient (96.91%), EXT + HA to patient (96.43%), EXT + MSCs to MSCs (72.26%), EXT + MSCs to HA (106.96%) and EXT + HA groups increased significantly compared to HA (97.90%) (Figure 2).
The mean and deviation criteria related to DKK1 gene expression in cardiac tissue in different studied groups are shown in Figure 3. Significant differences in the mean and standard deviation of DKK1 gene expression in cardiac tissue were observed between different groups (P < 0.000).
The results of the ANOVA, calculated F value (15/832) and its significance at P = 0.000, indicated a significant difference in DKK1 gene expression in cardiac tissue between different research groups. Based on these findings, the average DKK1 gene expression in cardiac tissue in osteoarthritis mice was significantly reduced in the patient, saline, HA, MSCs, and EXT groups compared to the other groups. The other groups showed a significant increase compared to the control group. Accordingly, the EXT + MSCs to patient (99.99%), EXT + MSCs to EXT + HA (96.62%), EXT + MSCs to MSCs + HA (91.31%), EXT + MSCs to EXT (97.65%), EXT + MSCs versus MSCs (99.86%), and EXT + MSCs groups had a significant increase compared to HA (99.91%) (Figure 3).
The mean and deviation criteria for Fz gene expression in cardiac tissue in different studied groups are shown in Figure 4. Significant differences were observed in the mean and standard deviation of Fz gene expression in cardiac tissue between different groups (P < 0.000).
The results of the ANOVA test, calculated F value (22/387) and its significance at P = 0.000, indicated a significant difference in Fz gene expression in cardiac tissue between different research groups. Based on these findings, the average Fz gene expression in cardiac tissue in osteoarthritis mice increased significantly in the patient, saline, HA, and MSCs groups compared to the other groups. The other groups showed a significant decrease compared to the control group. Accordingly, the EXT + MSCs to patient (91.97%), EXT + HA to the patient (75.40%), EXT + MSCs to MSCs (81.21%), EXT + MSCs to HA (81.23%), and MSCs + HA groups decreased significantly relative to the patient (78.91%), EXT to the patient (78.91), MSCs to patient (57.26), and HA to the patient (57.22%) (Figure 4).
The mean and deviation criteria related to Wnt gene expression in cardiac tissue in different studied groups are shown in Figure 5. Significant differences in the mean and standard deviation of Wnt gene expression in cardiac tissue were observed between different groups (P < 0.000).
The results of the ANOVA test, F value (10.309), and P-value = 0.000 indicated a significant difference in Wnt gene expression levels in cardiac tissue between different groups. The findings showed that the average Wnt gene expression in cardiac tissue in osteoarthritis mice increased significantly in the patient, saline, HA, and MSCs groups compared to the other groups. The other groups showed a significant decrease compared to the control group. Accordingly, the EXT + cell to patient (69.82%), EXT + HA to the patient (49.07%), EXT to the patient (35.61%), MSCs + HA to the patient (41.19%), and EXT + MSCs groups had a significant decrease compared to MSCs (60.37%), EXT + MSCs compared to HA (60.59%), EXT + MSCs compared to EXT (53.12%) (Figure 5).
The mean and deviation criteria for TCF gene expression in cardiac tissue in different studied groups are shown in Figure 6. Significant differences were observed in the mean and standard deviation of TCF gene expression in cardiac tissue between different groups (P < 0.000).
The results of the ANOVA test, calculated F value (23.436) and its significance at P = 0.000, indicated a significant difference in TCF gene expression in cardiac tissue between different research groups. Based on these findings, the average TCF gene expression in heart tissue in osteoarthritis mice increased significantly in the patient and saline groups compared to the other groups. The other groups showed a significant decrease compared to the control group. Accordingly, the exercise + cell to patient (91.97%), exercise + HA to patient (87.70%), cell + HA to patient (81.67%), EXT to patient (78.92%), MSCs to ratio patient (52.27%), HA relative to patient (57.22%), EXT + MSCs versus MSCs (81.21%) and EXT + MSCs groups had a significant decrease compared to HA (81.46%) (Figure 6).
3.1. Histological Considerations
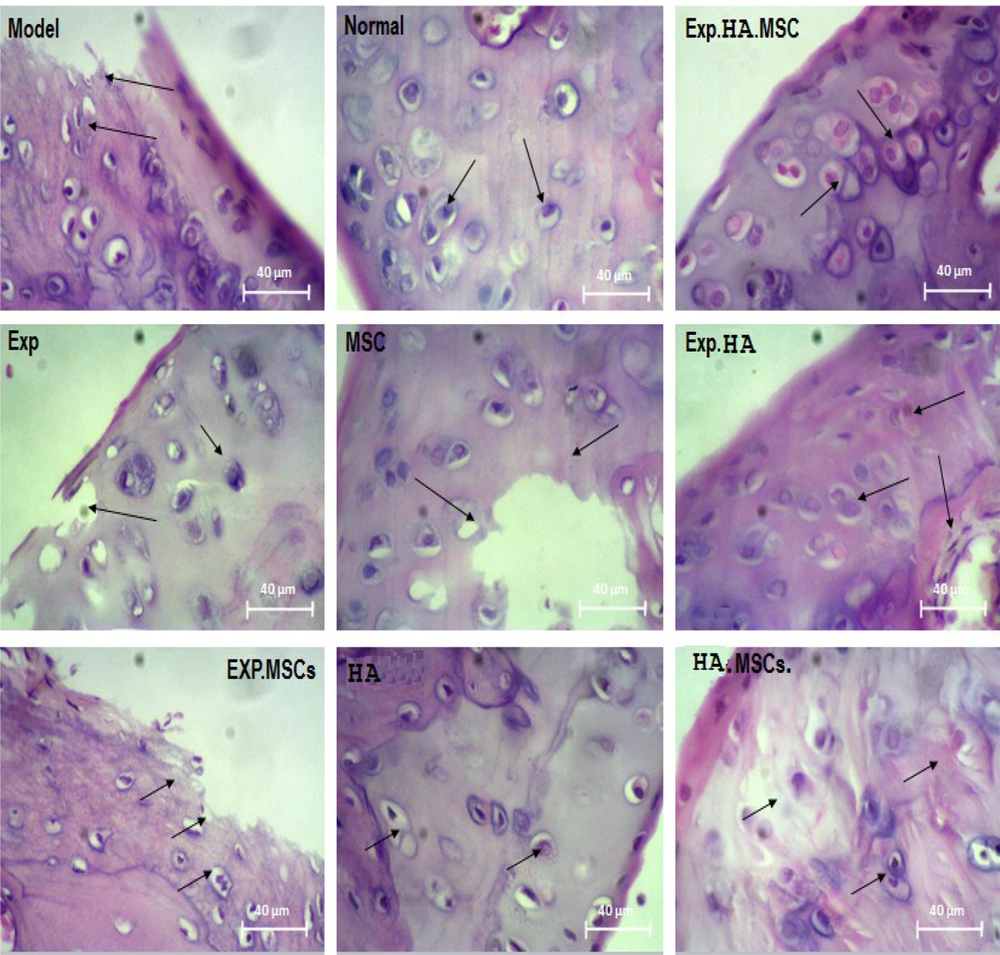
The results of H&E staining in the knee tissue showed that the chondrocyte cells were damaged in the rats' knee tissue in the osteoarthritis injury model. Moreover, the destruction ratio in the synovial membrane, the lateral ligaments, and the retaining muscles was observed on the induction side of the model. According to the observed results, the destruction of bone tissue in the area below the articular cartilage was clearly evaluated. On the other hand, the isogenic groups were observed to a small extent in this group. The groups involved the accumulation site of several chondrocytes in a cavity and indicated the proliferation of cartilage-forming cells. In the normal group, regular rows of chondroblast and chondrocyte cells were observed in the cavities as isogenic groups. Additionally, the border between each cartilage cell and the extracellular matrix was seen as a colored halo around the cell, showing the presence of glycoproteins around the chondrocyte cavities. The bone tissue below the articular cartilage area was observed as normal and in the form of some cavities containing bone marrow tissue, with a regular arrangement of osteocyte cells in a consistent manner. Other joint structures, including the synovial membrane, the lateral ligaments, the supporting muscles around the joint, and the joint capsule, were normal. In the cohort of patients with concomitant exercise therapy, the cartilage formation ratio was significantly improved in such HA receiving groups. However, in some areas, the collagen fibers were replaced by scar tissue instead of the normal cartilage tissue. This group showed a better improvement trend than the group receiving MSCs and EXT. In the MSCs and HA groups alone, the unrepaired areas of cartilage and some parts of the bone were still abnormal in some areas. However, the improvement process was better in the HA group than in the MSCs injection group. A recovery process similar to that in the MSCs group was observed in the EXT group. According to the results achieved in the MSCs, HA, and EXT groups, the improvement process was better in the HA group than in the other groups. The combined group of EXT, MSCs, and HA had the best results compared to the other therapeutic groups. Thus, the proliferating cartilaginous cells inside the lacunae were seen as isogenic groups, which were even more than the healthy group. In the cartilaginous region of the bone tissue, the damaged cartilaginous areas were completely replaced with the bone tissue. The articular capsule and the lateral medial ligament were well repaired from the lateral cavities (Figure 7).
4. Discussion
The OA induction in the rats led to a significant increment in the β-catenin, Fz, Wnt, and TCF genes. It also led to a significant decrement of the DKK1 and GSK3 genes in cardiac tissue. The significant differences compared to the control group indicated activation of the signaling pathway Wnt/β-catenin in the heart. Previous studies have shown that activation is common between OA and CVD (29). In a study by Lietman et al. Wnt signaling inhibitors improved OA in the experimental model (13). Also, Zhao et al. showed that Wnt signaling played a key role in hypertension, cardiac hypertrophy, and cardiac fibrosis (12).
On the other hand, our results confirmed that the EXT, EXT + HA, and EXT + MSCs groups led to a significant decrement of the genes β-catenin, Fz, Wnt, and TCF, but a significant increment of the DKK1 and GSK3 genes. The heart tissue was compared with the patient group, where the changes in the EXT group were not significant alone but were significant in the EXT groups in combination with HA and MSCs. Also, HA and MSCs did not alter the β-catenin and GSK3 genes of the heart tissue.
Today, intra-articular injection of HA is widely used in the treatment of OA and pain relief (26). The mechanism of action in HA includes increasing the number of live chondrocytes, creating thickness and repair at the cartilage level, preventing nitric oxide production in synovial fluid and meniscus, inhibiting chondrocyte apoptosis, and reducing MMP-3 and IL-1β in synovial fluid (30-32). Moreover, the combination of HA with EXT had a better effect on inhibiting Wnt signaling in the patient's heart tissue. It may be due to the same mechanisms, the changes of HA in the knee structure, or the reduced pain in the subjects. Definitive commentary requires further study in this area.
In OA, the existing MSCs are degraded. Moreover, their capacity increases but their ability to differentiate decreases. Therefore, systemic or topical administration of stem cells to these individuals can increase the repair or inhibition of joint tissue loss. It is because the main function of stem cells is to regenerate and repair damaged and old tissues (33, 34). Besides, MSCs are a good choice for therapeutic usage due to their low immunogenicity and regulatory effects on the immune system (35). Our results have the same positive effects because the combination with EXT, compared to the patient group, led to inhibition of Wnt signaling in cardiac tissue. In Amyloid-β-treated neural progenitor cells (NPCs), the cultivation of MCSs significantly increased the expression of Ki-67, GFAP, SOX2, nestin, and HuD compared to the Aβ group alone. Treatment of MCSs in Aβ-treated NPCs, in comparison to the Aβ group alone, only increased the expression of β-catenin and Ngn1 (36). MSCs and EXT had a synergistic effect on inhibiting Wnt signaling and led to the best results in this group.
The neuromuscular and Wnt signaling pathways are involved in Aβ-related AD models. A study in vivo by Zheng et al. (36) showed that Aβ inhibited neurogenesis of the hippocampus in the adult brain by regulating interferon-G depletion and NF-kB transcription. Other animal studies have supported the role of neuroinflammation in the neurogenesis of the hippocampus by showing that the regulation of microglia activity or neuropathy is associated with the regulation of neurogenic activity in the hippocampus of Alzheimer's patients (37, 38). He and Shen (39) showed that amyloid-β could regulate the neurogenesis of the hippocampus by interrupting β-catenin signaling, thereby increasing amyloid-β leading to the induction of GSK-3β, which promotes phosphorylation and β-catenin degradation. The Wnt/β-catenin signaling pathway plays a key role in regulating the differentiation and proliferation of neural stem cells.
One study found that MCSs enhanced neurogenesis and provided neurological differentiation against amyloid-β toxicity in experimental Alzheimer's disease models by adjusting the Wnt signaling pathway (36). Treatment of MCSs in NPCs treated with amyloid-β significantly increased the expression of β-catenins and Ngn1, which was attenuated by amyloid therapy, leading to an increase in the number of NPCs and positive BrdU cells in Alzheimer's disease animal and cellular models (36).
Regarding the precise molecular mechanisms of MCSs that underlie the Wnt signaling pathway, some recent prophylaxis studies have shown that MCSs express different proteins associated with the Wnt signaling pathway (40, 41). Also, Salazar et al. (42) found that MCSs derived from bone marrow and umbilical cord blood biologically produced active Wnt proteins. Moreover, the reproductive effects of MCSs decrease when inhibited by a Wnt signaling antagonist. Accordingly, the increment of Wnt proteins produced by the MCSs treatment may neutralize the negative effect of ABB on neurogenic activity. As a result, AB binds to the Fz range rich in Wnt receptor cysteine and inhibits the Wnt/BB-catenin signaling pathway (43, 44).
Sadeghiehpour et al. investigated the effect of six weeks of endurance training on the expression of the GSK-3β gene in the ranks of diabetic neuropathy. They found that the expression of this gene, as a result of endurance training in the group of exercise diabetes decreased compared to the group of diabetes. This reduction has been significant and may have been due to adaptation to endurance training (45). The research results showed a significant relationship between changes in GSK-3β expression in skeletal and cardiac muscle of diabetic mice and exercise. Some studies have shown a reduction in this kinase by performing exercise in twin muscles of rats. The studies reported that this decrease was probably due to increased activity in Akt/mTOR messaging pathways, which are phosphorylation pathways and GSK-3β inactivators (45).
Furthermore, the analysis of research data from Sadeghiehpour et al. (45) showed that the expression of the GSK-3β gene in the diabetes insemination group significantly increased compared to the healthy control group. This increment was probably due to neurological damage caused by diabetic neuropathy. GSK-3β is involved in the messaging, survival and development of neurons (46), the expansion of dendrites, and the formation of synapses in newborn neurons (47). Consequently, this kinase is critical in neurons, and GSK-3 acts as a regulator if there is a regulatory pattern in the pathways. It will witness the development of neurodegenerative diseases such as diabetes, Alzheimer's (48), and other motor neurological disorders such as amyotrophic lateral atherosclerosis (ALS) (45). Also, studies on the association of GSK-3β with Alzheimer's disease show that GSK-3 is involved in the production of beta-amyloid plaques that can be manipulated by GSK-3 to achieve an acceptable treatment strategy in the fight against Alzheimer's (49). Additionally, it has been shown that the inhibition of GSK-3 can be a therapeutic strategy for ALS, which delays the onset of symptoms and death (45). GSK-3β gene expression was lower in the exercise diabetes group than in the healthy control group, although not significantly. Exercise has been shown to moderate the expression of this gene in the group with neuropathy of diabetes. It also reduces the expression of this gene to its normal limits.
Stranahan et al. reported the impact of changes in Wnt gene expression in the hippocampus and Wnt signaling patterns on memory, learning, and flexibility of the hippocampus after voluntary exercise (50). Hence, Bayod et al. focused on the Wnt focal signaling pathway as a molecular pathway that can be adjusted in the hippocampus after moderate long-term exercise and environmental enrichment. The animals were touched and exposed to unmoving treadmills for the same amount of time as the training group. Bayod et al. and other researchers found similar results in middle-aged rats after 8 months of treadmill training, such as improvement in the age-related spatial learning and cellular mechanisms associated with flexibility and growth factor expression (51).
When the Wnt pathway is activated, β-catenin is transferred to the nucleus, where it activates the expression of genes involved in regulating homeostasis and neuronal survival (52). On the other hand, the degradation complex binds to β-catenin and phosphorylates it. Then, it targets β-catenin for ubiquitination and proteinase-related degradation. In this regard, some studies in vivo have reported the decrement of the Wnt/β-catenin signaling regulation that destroys neurons in the hippocampus (53).
DKK-1 is one of the most famous and well-studied Wnt antagonists. The antagonist has received growing attention in recent studies. It has been identified as a component of the sequence of events in neural death in different individuals for many years. Many in vitro and in vivo studies have reported an increase in the expression of DKK-1 in various disease models (54).
Seib et al. (55) indicated that reducing DKK-1 expression could counteract the age-related reduction in neurogenesis and a related cognitive decline. In a study by Bayod et al. the DKK-1 immunohistochemical analysis showed a stronger signal in the sinusoidal brain of immobile mice than in trained mice, albeit the trained mice had less immunodeficiency for DKK-1. Additionally, the western blotting increased DKK-1 protein levels in sedentary animals compared to the trained animals (51). Also, total Fz protein levels were lower in sedentary mice than in the trained group. It could be related to the degradation and internalization of DKK-1 derived from the Fz receptor (51, 55).
Bayod et al. analyzed some compounds in the degradation complex such as Axin1 and GSK-3α/β. Regarding DKK-1, higher levels of Axin1 and increased activation of GSK-3α/β were measured in sedentary animals according to their phosphorylation at Tyr279/216. Therefore, it is believed that Axin1 may facilitate β-catenin phosphorylation by GSK-3 in active mice, thereby targeting it for ubiquitination and protease degradation. On the other hand, a decrease in Axin1 levels and GSK-3 activation showed an increase in β-catenin nucleus transport and Wnt pathway activation in the trained mice. This led to the nervous survival of these animals. Therefore, the different phosphorylated forms of β-catenin and total β-catenin protein levels were not different between the groups. Furthermore, Rosi et al. (54) found no change in the β-catenin of the entire hippocampal extract. It deserves to note that the small changes in β-catenin protein levels can be related to critical tissue phenotypes associated with β-catenin signaling. In the study by Bayod et al. moderate physical activity may only cause slight changes in total phosphorylated β-catenin protein levels, which may be sufficient to activate the Wnt pathway (51).
4.1. Conclusions
The present study's findings showed that the induction of the experimental model of OA was associated with increased expression of the β-catenin, Fz, Wnt, and TCF genes and a significant decrease in DKK1 and GSK3 genes of cardiac tissue and activation of Wnt signaling, which may lead to CVD. Moreover, we found that regular exercise combined with HA and MSCs may reduce β-catenin, Fz, Wnt, and TCF expression, increase DKK1 and GSK3 in cardiac tissue, and inhibit Wnt signaling in the heart. This may have a protective effect and prevent CVD in the experimental model of OA. The best results were obtained in the exercise group combined with HA and MSCs.
![Relative GSK expression for different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group; $, significant symptoms on EXT + hyaluronic acid (HA)]. Relative GSK expression for different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group; $, significant symptoms on EXT + hyaluronic acid (HA)].](https://services.brieflands.com/cdn/serve/3170b/4dd96243eda680b5077e1ce01f7fc218f0af4f1e/jamm-122228-i002-F2-preview.webp)
![Relative DKK1 expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group]. Relative DKK1 expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group].](https://services.brieflands.com/cdn/serve/3170b/b43d6d99858569141e9cf4e6dc8ffdbd1613b507/jamm-122228-i003-F3-preview.webp)
![Relative FZ expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group]. Relative FZ expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group].](https://services.brieflands.com/cdn/serve/3170b/cca299a6e1e31e4c821cccbcb3d7ada69b5c0ae0/jamm-122228-i004-F4-preview.webp)
![Relative WNT expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group]. Relative WNT expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group].](https://services.brieflands.com/cdn/serve/3170b/1cff1d111be27abdeb714759114b6de99bb087ac/jamm-122228-i005-F5-preview.webp)
![Relative TCF expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group]. Relative TCF expression in different research groups [*, significant symptoms compared to the patient group; &, significant sign of the EXT + mesenchymal stem cells (MSCs) group].](https://services.brieflands.com/cdn/serve/3170b/917861772d5e11f403debd0a80e352ea595c68fa/jamm-122228-i006-F6-preview.webp)