1. Background
Prions are transmissible proteinaceous infectious agents that cause spongiform encephalopathies resulting in degenerative neurological disorders (1). In humans, on chromosome 20, lays the PrNP gene-producing the PrP protein (2). Prions cause an abnormal isoform of this PrP protein resulting in the misfolded disease-causing PrPsc protein. Prion disease is characterized by an extended incubation period as the PrPsc isoforms accumulate in the neural cells. There is a slow loss of neuronal cell function and failure to induce an inflammatory response, resulting in a spongiform encephalopathy and ultimately death (3).
Clinical features rarely present in young patients unless direct exposure has been encountered. CJD usually appears in older populations with death occurring within six months from the onset of symptoms. There are currently no curative treatments for CJD or other spongiform encephalopathies (1). Initial signs of CJD include cognitive decline, seizures, sensory deficits, visual abnormalities, and extrapyramidal symptoms (2). The early signs of CJD are often congruent with other diseases such as Parkinson’s and Alzheimer’s, which makes CJD commonly misdiagnosed (4).
The primary prion diseases which affect humans are Kuru, Creutzfeldt-Jakob disease (CJD), Gerstmann-Sträussler-Scheinker syndrome (GSS), and fatal familial insomnia (FFI), iatrogenic Creutzfeldt - Jakob disease (iCJD), and variant Creutzfeldt - Jakob disease (vCJD) (3). Additionally, prion disease spares no expense on species’ inflicted. Bovine develop bovine spongiform encephalopathy (BSE), otherwise known as mad cow disease. Goats and sheep develop scrapie. Mink disease is described as transmissible mink encephalopathy. Mule and deer develop chronic wasting disease (CWD). Recently, domesticated cats have been found to have feline spongiform encephalopathy. Lastly, prion disease has been found several zoo species documented as spongiform encephalopathies of captive zoo animals (5) (Table 1).
| Disease | Abbreviation | Host |
|---|---|---|
| Creutzfeldt-jakob disease | CJD | Human |
| Variant creutzfeldt-jakob disease | vCJD | Human |
| Iatrogenic creutzfeldt-jakob disease | iCJD | Human |
| Bovine spongiform encephalopathy | BSE | Cattle |
| Kuru | Kuru | Human |
| Gertsmann-straussler-scheinker disease | GSS | Human |
| Fatal familial insomnia | FFI | Human |
| Scrapie | Scrapie | Goat, Sheep |
| Transmissible mink encephalopathy | TME | Mink |
| Chronic wasting disease | CWD | Mule deer, Elk |
| Feline spongiform encephalopathy | FSE | Cats (domesticated) |
| Spongiform encephalopathies of captive zoo animals | 12 species zoo animals identified |
While CJD is rare, iCJD is unique in that this form is transmitted through contaminated tissue typically during medical procedures (6). Iatrogenic transmission of CJD has occurred from exposure to infectious brain tissue, dura matter, pituitary tissue, and eye tissue in approximately 1% of CJD cases (2). Iatrogenic CJD has been described in three circumstances: contaminated surgical equipment, after the use of extracted pituitary hormone, and contaminated implanted human grafts (7). The transmission via stereotactic electrodes is a possible route of transmission because of the proximity to brain tissue and the inability to properly sterilize this medical device (4). Currently, it is speculated that more than 492 cases have been identified worldwide (8).
Current literature has no evidence of transmission through regular social interaction or nursing contact (9). Transmission may occur when tissue or body fluids come in contact with the infectious agent after incomplete sterilization (4).
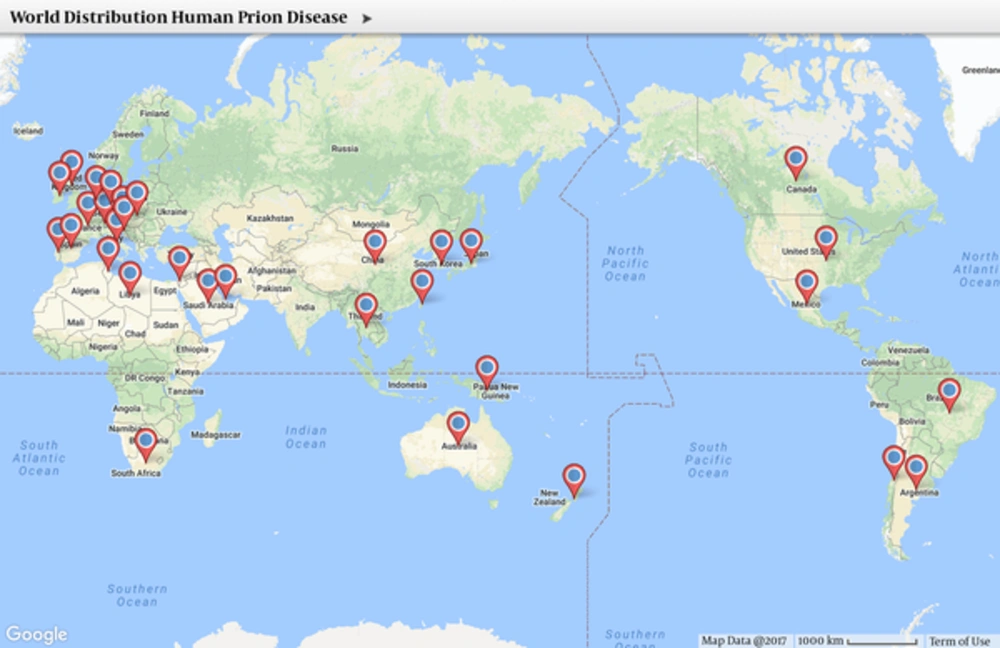
Long incubation periods make it hard to predict an epidemic curve (5). Figure 1 displays a geographic distribution of human prion cases globally. Prion surveillance requires tests that determine the causative agent and ability to diagnose new cases. The definitive diagnosis of CJD comes from histological pathology from infected tissue (10), usually postmortem. Invasive biopsies are the only way to diagnose prion disease through detection of PrPsc definitively (11).
In 2011, the novel assay real-time quaking-induced conversion (RT-QuIC) developed by microbiologist Ryuichiro Atarashi was used to test for prion disease in mouse models (12). It was found to have 100% specificity and 83% sensitivity. In a recent publication by Foutz et al. (2017) the work from Atarashi progressed to test the assay on over 2000 patients with degenerative neurological conditions in Australia. The measurement of prion seeding activity with RT-QuIC found that it was a superior method to current diagnostic techniques with 95% sensitivity and 100% specificity (11). RT-QuIC also has a fast turn-around time of 48 hours. Current practice utilizing surrogate markers for 14 - 3 - 3 and tau proteins from biopsy samples are invasive, slow diagnostic times and high rates of false-positives (12). RT-QuIC is now available at the national prion disease surveillance center in Cleveland, OH (11). This method is being identified as a potential opportunity to test blood to ensure blood and tissue products are clear of prions before transfusions and transplants.
The United States department of defense currently has a program in place, the national prion research project (NPRP), which has established a research effort to accelerate the capabilities to prevent or ameliorate prion disease (5). The global health and economic catastrophes caused by prion disease have preemptively triggered attempts to avoid the widespread dissemination of the disease among animals, especially those ingested by humans. Additionally, the United States department of agriculture (USDA) has established a national scrapie eradication program to prevent scrapie’s infected meat from entering the United States (13). Although scrapie’s has not been shown to infect humans directly, it is thought to be the route for the development of BSE in cattle. BSE in cattle has been confirmed to cross species barriers and cause vCJD.
Prion disease has been shown to cross species, with the most significant risk coming from ingesting the tissue infected with prion particles. Kuru was seen in the Fore People of Papua New Guinea who practiced cannibalism. Kuru occurred despite the human meat having been cooked, proving it to be resilient to high temperatures without affecting its infectious nature (14). Subsequently, novel research has demonstrated that prions can be transmitted from infected waste into the sewage system and has the potential to infect water sources (15). Wastewater plants are also ill-equipped and unable to deactivate prion proteins (15).
2. Epidemiology
The U.S deploys to more than 144 countries where vCJD has been reported. Not only are soldiers deployed, but are frequently accompanied by their families. Possible exposure to bovine spongiform encephalopathy (BSE) stock can occur to those deployed families. Currently, military policies prohibit purchasing beef from any country reporting cases of BSE. Beef purchases from all countries must be approved by the DOD due to the risk of exposure to prions. Table 2 provides a list of countries with confirmed BSE cases from 2006 - 2016 and the presence of US military in those countries.
| Country | 10-Year Case History | Military Presence |
|---|---|---|
| United Kingdom | 206 | Yes |
| Austria | 15 | |
| Brazil | 2 | Yes |
| Canada | 16 | Yes |
| Belgium | 2 | Yes |
| Czech Republic | 7 | |
| Denmark | 1 | |
| France | 50 | Yes |
| Germany | 26 | Yes |
| Ireland | 189 | |
| Italy | 12 | Yes |
| Japan | 15 | Yes |
| Netherlands | 8 | Yes |
| Norway | 1 | Yes |
| Poland | 35 | Yes |
| Portugal | 87 | Yes |
| Romania | 2 | Yes |
| Slovakia | 2 | |
| Slovenia | 3 | |
| Spain | 176 | Yes |
| Sweden | 1 | |
| Switzerland | 8 | |
| United States | 2 | Yes |
The risk of transmissible spongiform encephalopathy (TSE) from blood products (blood transfusions) may also place deployed forces at risk of infection (16). DOD blood supply is under the management of the armed services blood program (ASBP) which is closely monitored with six collection sites overseas (17). Even though the risk is low for TSE transmission, blood donations are not accepted (for life) from those individuals who reside in countries with active prion disease outbreaks. In 2003, vCJD was reported in 5 cases that received blood transfusions from donors who subsequently developed vCJD (18). These infections are evidence that assays need to be available to screen blood products more readily. There is an increase in the incidence of BSE around Europe and Japan that are not linked to imported cattle. Aside from the United Kingdom- Portugal, Ireland, and Switzerland have the highest cases of prion disease. BSE in the United States has only resulted from imported cattle already infected (19).
Recently, feline spongiform encephalopathy has been described in domesticated felines and a few species in zoos throughout the United Kingdom and an isolated case in Australia. The emergence in household animals and animals in zoos further present a public health concern that vCJD has the possibility of a resurgence as was seen in the 1990’s (20). Although FSE has not been shown to cross species barriers, there has been a documented case of a possible man-feline transmission of vCJD. Genetic analysis revealed similarities between the species of prions isolated from the human and the cat (20).
Chronic wasting disease (CWD) has also presented itself as a concern to North America. CWD is not thought to cross species barriers, but environmental protection agencies recommend hunters not to consume deer, elk, or moose meat (specifically spleen, brain, spinal cord) that appear sick or acting abnormally (13).
3. Infection Control Issues Associated with Prion Disease
Only a small number of prion disease cases have been associated with iatrogenic transmission (6). However, the financial and social cost associated with these infections cannot be ignored. The loss of public trust in medical institutions, investigative costs, lawsuits, etc. poses a risk to the infrastructure of organizations and medical professionals (21). Prion resistance to common decontamination techniques makes present-day hospital sterilization processing a challenge. The difficulty in establishing the likelihood of cross-transmission stems from the lack of early diagnostic tools in assessing for prion disease (historically postmortem). Additionally, cross-contamination theory is extrapolated from studies on prion inactivation using mouse models, epidemiological data on transmission rates, infectivity of human tissue, and the ability of current cleaning standards to remove microbes. Surgical equipment and devices that come into contact with high-risk tissues such as the brain, spinal cord, and eyes should have additional protocols in place that offer prion-specific sterilization and decontamination (4).
Prion diseases, such as Creutzfeldt- Jacob Disease offers a unique infection control problem because of their resistance to conventional physical (radiation), chemical, enzymatic, and thermal decontamination methods and maintain their stability in the environment for years (22). With a rise in interest from the scientific and public health sects, the potential public health concern around CJD is no longer limited to hospital settings (5).
Currently, most disinfectants, especially those that act on nucleic acids, are unable to deactivate prions (23). In cases where there is a substantial infectious load, steam autoclaving at 134°C for 18 minutes (10), cannot guarantee complete inactivation. Prions are tenacious which makes it essential to establish prophylactic measures, specific cleaning procedures, and sterilization/disinfecting methods to minimize the risk of iatrogenic transmission. The lack of early diagnostic tools makes it difficult for rapid diagnosis making it difficult to institute appropriate infection control measures in suspected case. Additionally, most hospital protocols are not set up to monitor what surgical equipment issued to each patient making it nearly impossible to isolate patients that may have been exposed to prion exposed hardware even after disinfection. Surgical equipment and devices that come into contact with high-risk tissues such as the brain, spinal cord, and eyes should have additional protocols in place that offer prion-specific sterilization and decontamination (4).
The assays that have been used to detect residual PrPsc on stainless steel after exposure to infected tissue are histopathology, immunohistochemistry, western blot, scanning electron microscopy, and protein analysis (10, 22, 24). The result from the experiments demonstrated that decontamination of surgical equipment, i.e., stainless steel, remains an issue (24). In fact, prion proteins were harder to remove from stainless steel surfaces than other surfaces that were analyzed especially after the tissue had dried to the surface (22). Stainless steel equipment after exposure to infectious material showed contamination in 100% of all apparatus (24). Prion proteins were not able to be washed off under normal cleaning conditions. This data suggests that contaminated surgical equipment needs to have additional sterilization techniques in place to ensure there is no nosocomial spread of prion disease.
Chemical and thermal deactivation has been analyzed by several authors to establish best inactivation methods. Prions can resist deactivation by formalin and other aldehydes (23). Prions incubated in Sodium hypochlorite for 30 minutes were no longer infective. However, sodium dichloroisocyanurate containing a similar concentration of chlorine was not effective at deactivating the infectious agent (10). Sodium Hydroxide was once believed to disinfect prions, but that was in trials diluting infectious samples. When samples were undiluted sodium hydroxide has no impact on infectivity (22). Refer to Table 3 for the efficacy of common disinfecting practices against prion seeding activity.
| Optimal Method of Decontamination | Suboptimal | Not Acceptable |
|---|---|---|
| Sodium Hypochlorite (1 hour) | 1 or 2 M Sodium Hydroxide (1 hour) | Hydrogen peroxide, aldehydes, HCl, ClO2 |
| Autoclaving at 121°C after Sodium Hydroxide treatment | Prolonged autoclaving 1 - 4 hours @ 132°C | Phenolic disinfectants, iodophors, alcohols |
| AcOH and + synthetic polymer | Extended steam sterilization with 1M NaOCl | Proteolytic enzymes |
| Incineration | Dry Heat > 200°C | Radiation, UV, microwave radiation |
| Paracetic acid-base + buffers, anticorrosives, surfactants, and chelators liquid mix @ 50 - 56°C for 12 minutes X 2 passes. | Porous load autoclaving at 134° - 138°C | Biocides |
| Gaseous hydrogen peroxide under vacuum conditions | Gaseous hydrogen peroxide under atmospheric conditions with enzymatic pre-cleaning |
Additional studies were able to demonstrate that weak acids like acetic acid (AcOH) in combination with synthetic polymers were able to render prions susceptible to proteolytic degradation (23). Sodium dodecyl in conjunction with AcOH was a potent combination in deactivating the protein (23). Table 3 illustrates the different chemical agents that have been tested and their success in deactivating the prion protein.
Heat deactivation has also been studied to optimize reduction infectivity. Researchers have exposed different infected samples to a range of heat and time intervals and evaluated the infectivity and structural integrity of the protein. Times ranges from 15 minutes at 600°C to 24 hours at 160°C (24). All heats and time intervals were unable to inactivate the protein completely. At 600°C, the protein structure was retained in an inorganic fossilized skeleton which was still able to convert PrP into PrPsc (24). Finally, gaseous hydrogen peroxide under vacuum conditions has been shown to be most effective in eliminating prion proteins from hardware (25).
4. Conclusion
Prion diseases are rare and do not constitute a significant infection control risk. However, iCJD has now been shown to be transmitted through tissue transplants, blood transfusions, and contaminated surgical equipment (6-8). Because, of their affinity for stainless steel and superior resistance to current sterilization techniques used on surgical equipment, novel approaches for deactivation of prions should be addressed in the prevention of spreading nosocomial infections (24). Staying up-to-date on novel diagnostic tools available for the detection of prion seeding activity will also assist in preventing transmission.
For military bases and hospitals that may have limited resources overseas, it is imperative to design new technologies that allow for antemortem diagnoses in patients presenting differential diagnoses, prion disease not being excluded, as well as for animals in countries with BSE outbreaks. New technologies will allow for better protection of military personnel, preventing nosocomial and foodborne transmission.
Changes to the current infection control practices would require substantial changes if prion infection is suspected. These would include the methods discussed above and in Table 3.
5. Recommendations for Prospective Research for the Military in Prion Disease
1. An antemortem laboratory test to detect PrPc with the capability to detect less than one infectious unit (IU) of prion seeding per sample. Approximately 1 IU is equivalent to 105 PrPSc molecules in a purified preparation depending on the host, strain, and mode of transmission (15). Utilizing the current RT-QuIC assay overseas and creating the ability to test blood samples will undoubtedly prevent iCJD transmission.
2. Development of novel molecules to detect and bind to prions for in-vitro studies. Antibodies and molecules specific to species, strain, and allelic variants for infectious conformation will allow for more accurate diagnosis. Selected antibodies specific for the conformation of prions are lacking because of immunogenicity to the PrPsc (15).
3. Cell culture technique improvement to allow for propagation of prions. In vitro cell cultures are lacking while transgenic mice often do not accurately represent the physiology of human pathogenesis and propagation of disease.
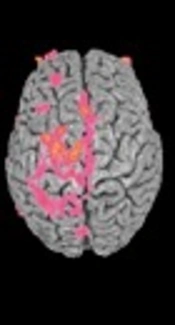
4. Refined neuroimaging techniques in conjunction with an MRI that will allow for the detection of PrPsc for early diagnosis (1).
5. Priority research initiatives need to identify direct and indirect routes of TSE transmission. Determine individual host susceptibility and resistance by continuing to investigate genetic susceptibility as well as exploring the epigenetic factors. A focus should also be placed on the host’s immune response to prion disease through the intracellular trafficking of PrP in different cells and identify a mechanism for neuroinvasion (1).