1. Cardiovascular Diseases in Public Health
Overall, 10% to 20% of heart attacks occur with no diabetes, hypertension, cholesterol or smoking (1). When stent or coronary angiography is installed, the patients are informed that they have stiff arteries, or familial arteriosclerosis. Stresses due to the pain of the heart attack, disability, and costs for public health in general and for the family are important issues. Nevertheless, today, there is no preventive action in the direction of families, while there exists non-invasive medical devices that can measure the aging of arteries in the general population.
Cardiovascular diseases (CVDs) remain the leading cause of deaths worldwide. Approximately 30% of all deaths consisting 20 million people will be caused by CVDs in 2015. Of these, an estimated 8 million are due to coronary heart disease and 7 million to stroke (2).
With aging, an increase of 50% in the rate of heart failure and stroke is expected for the next 25 years (3). Heart disease and stroke are the second cause of disability in the USA. People may live with heart disease for years or decades. It can severely limit their ability to work. Studies have estimated that heart disease is now the reason for 17% of all health costs in the US (4). Stroke costs are about 6.76 Billion € every year in France.
The economic burden related to the management of vascular diseases (heart attack, stroke, cognitive impairment, and heart and kidney failure) remains considerable. The direct and indirect costs are around 28 billion € every year in France.
Pulse wave velocity (PWV) is a very good surrogate for aging of the arteries (5). It has been studied for the past 40 years and it is now considered as an independent cardiovascular risk factor to be measured in hypertensive patients to detect silent organ damage. This was validated by the European society of hypertension and that of cardiology in 2013, and conducted in 2016: PWV is to be considered at the same level of proof of that of microalbuminuria in class IIa level B. Pulse Wave Velocity has a history of more than 25 longitudinal follow up studies for over 15 years, 4 meta-analyses with individual data, 200 European medical researches centers, and over 3000 scientific papers every year since 2012.
2. Classical Cardiovascular Risk Factors
The causes of cardiovascular disease were determined by prospective epidemiological studies, since 1950 (Framingham). In addition to high blood pressure, the 2 most frequent risk factors are diabetes and hypercholesterolemia. In hypertension, there is 28% of white coat syndrome and about 17% of masked hypertension this is about 45% of bad targeting (6, 7).
Several studies are based on data from the Framingham study, including systolic and diastolic blood pressure, aging (8), the low density lipoprotein (LDL)-cholesterol, triglycerides, high density lipoprotein (HDL)-cholesterol (9), hypertension (10), and PWV (11), which are all risk factors leading to cardiovascular disease.
A strong association has been found between the risk of cardiovascular event and alterations in the structure and/or parietal vascular function (4, 12). The stiffness of the vascular tree is physiologically related to aging in the aortic path that is constituted of 80% elastic fibers. The upper and lower limbs are muscular. Authors agree that arterial compliance is not correlated with age in limb arteries. The risk ratio of PWV is 2.3 while that of diabetes is 1.7; hypertension 1.8; cholesterol 2.0, and tobacco 1.9 (13). These are modifiable classical risk factors.
The non-modifiable risk factors are gender, age, and heredity. Risk markers are represented by psychosocial factors, stress, alcohol, C-reactive protein (CRP), fibrinogen, hyperhomocysteinemia, obesity, sedentarily, waist/hip ratio, dietary patterns, physical activity, consumption of alcohol, and blood apolipoproteins, which are known as confounding factors (14).
Pulse wave velocity assesses arterial stiffness. It is now admitted as an independent cardiovascular risk factor (15).
3. Arterial Aging, A New Risk Factor
3.1. Arterial Aging and Risk Factors
It is widely accepted that carotid-femoral pulse wave velocity (PWV) reflects Early vascular aging (EVA). Pulse wave velocity is an independent (5) cardiovascular risk factor that is better correlated with cardiac morbidity and all causes of mortality, than conventional risk factors, such as diabetes, hypertension, hypercholesterolemia, and tobacco intake (13, 16).
This was shown with large arteries stiffening using PWV. Local adverse effects of pulse pressure have not been validated (17). The interference between small arteries and large arteries can be linked to a virtuous circle; remodeling of small arteries and rarefactions of the vasculature that causes an increase in blood pressure, which increases the stiffness of the large vasculature with noble organ damage (heart, kidney, and brain).
The stiffness of large arteries increases pulsed blood pressure (systolic - diastolic blood pressure) and the heart work. Indeed, arteries provide a double role, including the transmission of the blood to the periphery and amortization of the cardiac pulsate pressure.
In fact, large arteries play a capacitive role in the vascular system; they restitute the pulsated energy in the diastolic time. In this diastolic time, coronary arteries will be supplied, and this will maintain diastolic pressure for a better coronary and brain blood supply, and more importantly, it will permit a continuous blood flow. The aorta constitutes of 80% of elastic tissue, which is why it plays a very important role in the vascular system and the amortizing of the blood pulse.
Different systems exist to assess the aging of arteries. Aortic aging can be evaluated directly by measuring the PWV, using different devices. Pulse wave velocity, measured between carotid and femoral sites, is considered as the gold standard of aortic central aging (18); it is associated with age, hypertension, diabetes, and cholesterol, and also with kidney, heart failure, and cognitive impairment.
The PWV is an important independent predictor of all causes of mortality, especially of cardiovascular morbidity, which has been demonstrated in patients with end stage kidney failure (19). Furthermore, PWV is an independent predictor of arterial stiffness morbidity and mortality in other types of patients with hypertension (5) or diabetes (20).
4. Factors Impacting AS
4.1. Gender
Pulse wave velocity is impacted by height yet not gender. In fact, people with short height were reported to have high PWV, since the velocity of the pulse wave is defined as the ratio of the distance between 2 measured sites to the time needed by the pulse to travel from one arterial site to another. This risk may be due to the short height of the aortic path of the pulse wave. Studies have shown that healthy females have a significantly higher PWV than males (21). This may be due to a higher stress reaction because of the location of the measurement in the groin. In our experience, there was no difference between males and females in the IPC cohort (health care center in Paris-France) using popmetre for PWV measurement (22). The majority of studies have found no association between PWV and gender (23).
4.2. Tobacco
Regarding other risk factors, tobacco is a known factor in the development and worsening of cardiovascular diseases. It significantly increases EVA, as measured by the PWV, because of its endothelial and vasoconstriction action (24). Regardless of age, measurements of PWV were significantly higher after smoking. Moreover, PWV is significantly higher in chronic smokers regardless of gender, general health, and physical activity levels. Passive smoking has detrimental effects and increases the risk of myocardial infarction. Recent studies have highlighted that some levels of exposure to smoking liabilities have a detrimental effect on AS, at a level slightly lower than active smoking. Other studies have demonstrated the impact of cigarette smoking on the stiffness of large arteries (25).
4.3. Obesity
Obesity has become a global epidemic in rich countries, both in children and in adults. Indeed, the prevalence of overweightness and obesity is more than 60% in adults of the USA and this rate increases in children and adolescents. Obesity is an independent risk for cardiovascular disease and is associated with other co-morbidities, such as type 2 diabetes, hypertension, and sleep apnea (26). It has been recognized that individuals with obesity are at a high risk of EVA, independently of brachial blood pressure, ethnicity, and age (27). Furthermore, it was found that central adiposity is a determining factor for arterial stiffening, independently of other factors such as age, and blood pressure. Similarly, it has been shown that aortic PWV is associated with obesity. Wildman et al. reported that median aortic PWV was 4 m/s higher in obese individuals than in normal weight subjects (28).
4.4. Cholesterol
High cholesterol levels are associated with high pulse pressure, aortic stiffness, and high central blood pressure, despite normal or low peripheral arterial blood pressure. In addition, LDL cholesterol is an independent risk factor for AS (29).
The decrease in serum cholesterol levels reduces the total cardiovascular mortality (30), by participating in the reduction of AS (31). Statins reduced aortic PWV over a period of 2 years. Simvastatin, associated with homocysteine and folic acid/vitamin B12, were
studied in a research trial (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine) to assess the effects of following an aggressive reduction of lipids. The analysis of the pulse wave was included in a sub-study of the research trial to assess the existence of the beneficial effect on AS (3). Recently, a meta-analysis confirmed these assumptions (32).
5. Arterial Stiffness and Age
With age, AS tends to increase, along systolic blood pressure and PWV. In particular, arterial stiffness measures the cumulative influence of CV risk factors with time, because age represents both the aging process and the duration of exposure to risk factors. Indeed, arterial stiffness represents true arterial wall damage, whereas other risk factors such as BP, glycaemia, and lipid levels vary during patient follow-up and thus may not be representative enough of the cumulative effects of CV risk factors on the arterial system.
A decline in coronary artery perfusion is observed with age, as well as increased risk of heart disease (33). The mechanisms of vascular impairment in elderly hypertensive patients look different from those of youth. With aging, the structural alteration of the arterial wall is at the forefront of physiological disorders. Aging is associated with PWV (34) or it could be said that with age structural alterations in the aorta appear (35).
Several studies have shown a positive relationship between PWV and age (36). Pulse wave velocity is more pronounced in individuals over 50 years (PWV increases 1 m/sec per decade before age 50, then 2 m/sec beyond). The increase of AS with age leads to an increased systolic pressure and pulse pressure (isolated systolic hypertension seniors).
6. Structural and Functional Rigidity
The increase in blood pressure is a factor of functional rigidity, while the increase of structural AS leads to increase in systolic blood pressure and pulse pressure. For a balloon, the increase in internal pressure leads to rigidity of the wall, while aging is the key for structural remodeling.
7. Role of Physical Exercise
It has been demonstrated that physical exercise decreases AS in patients with coronary artery disease (37) or end stage renal disease (38), and effectively reduces the risk of myocardial ischemia by decreasing myocardial oxygen demand and increasing coronary infusion.
In addition, physical exercises may impact AS related to normal aging. Individuals practicing endurance sports have lower AS than sedentary individuals of the same age and level of blood pressure. Reduced physical activity leads to a decrease in genetic expression
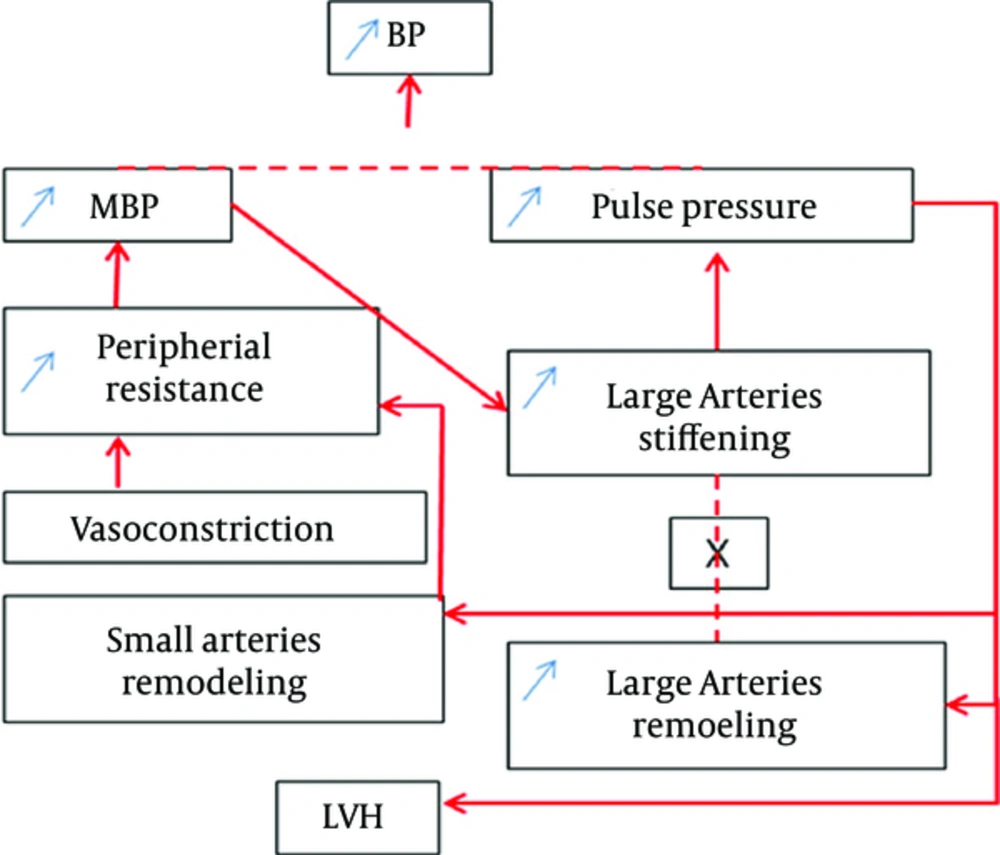
of predisposition to high systemic arterial stiffness (37). The interaction between large arteries and small arteries is illustrated in Figure 1 (39). The mean arterial pressure (MAP) and PP are remodeling factors for small and large arteries and the left ventricle. Arteriolar vasoconstriction is secondary to the increase in vasomotor tone, this increases peripheral resistances, leading to a high blood pressure. Arterial remodeling is a structural factor that increases peripheral resistance at the beginning of a real vicious cycle of increased stiffness and loss of compliance.
Arterial stiffening is an indirect result of aging and hypertension or other types of stress, such as chemical or oxidative stress. Studies have shown that PWV at the upper limb does not correlate with aging; a poor correlation may be found at the lower limb. However, when it comes to the aortic path, this correlation is very significant (40).
8. Mathematical Model of the Pulse Wave Velocity and Arteries Path
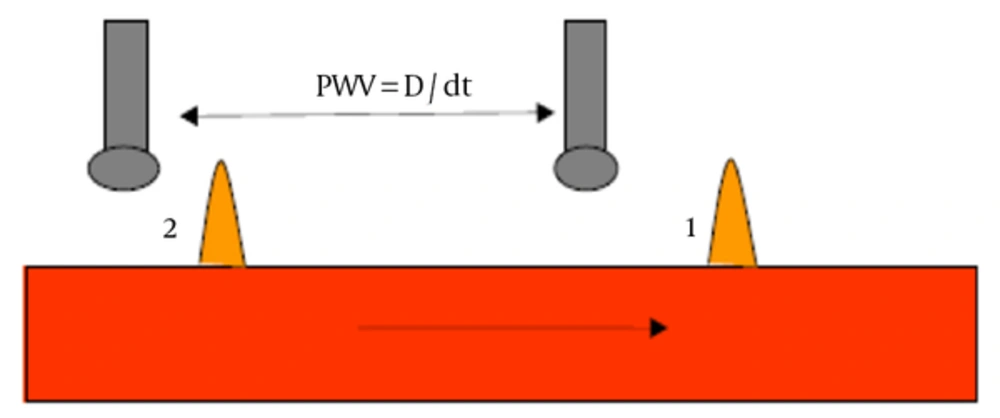
The PWV, defined as the travelled distance divided by the travel time, is measured using 2 similar pressure sensors located on the 2 sites of an arterial segment; the time needed by the pulse to travel from one arterial site to another and the distance between the measured sites was measured in this study (see Figure 2). The order of magnitude of the PWV varies between 5 to 15 m/s. It is different from the blood flow velocity in arteries, which is about 20 to 30 cm/s in large arteries. The aorta is the principal vessel of interest when measuring arterial stiffness because the thoracic and abdominal aorta are the principal sites for arterial buffering function, and aortic PWV has proved to be an independent predictor of outcome in various populations. However, all accessible arterial territories are potentially interesting. For instance, the forearm circulation corresponds to BP measurement, and the lower limb arteries are a classic site for atherosclerosis. The measurement of carotid stiffness also carries important prognostic information, because the carotid artery is also a possible site for atherosclerosis. Some devices measure arteries stiffening indices using transfer functions. These indices do not have any added value than that of brachial blood pressure measurement.
Pulse wave velocity depends on the visco-elastic characteristics of the arterial wall. It reflects the quality of the artery to “attenuate” the pulsate shock. Modeling the PWV has its origin in the works of Newton, who noted in a mathematical equation the relationship between velocity, elasticity, and density of the material.
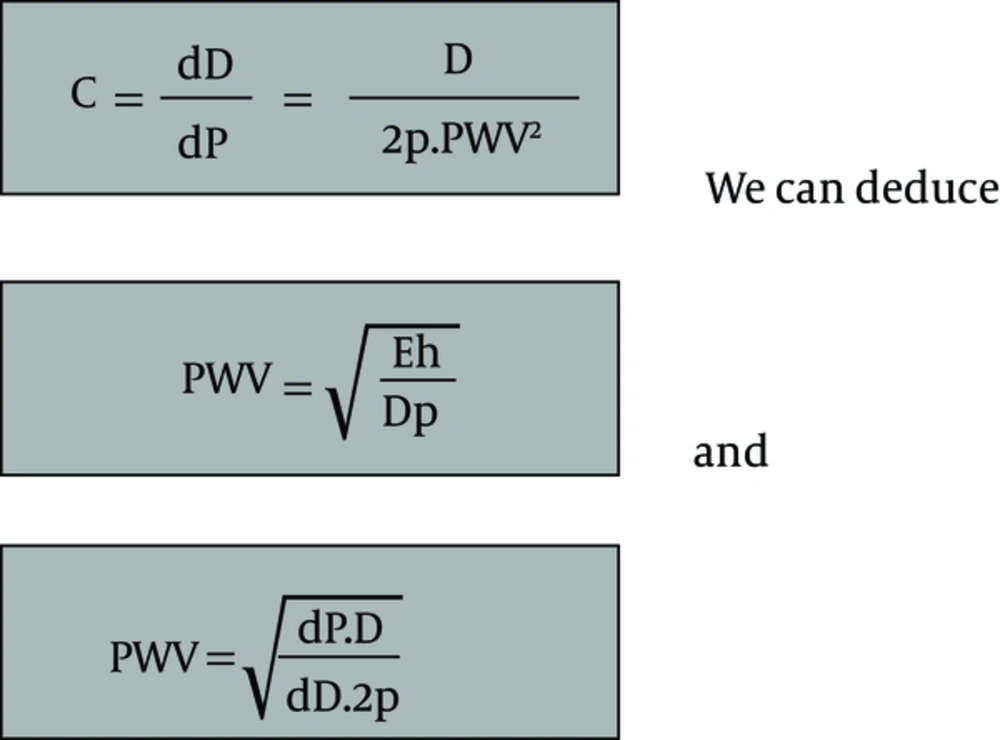
Thomas Young redefined PWV using the relationship between pressure variation and changes in volume for a given arterial segment. Moens and Korteweg worked on the modeling of PWV, according to pulse pressure and compliance (Figure 3), according to Armantano and Levenson (41). Pulse wave velocity is proportional to the variation of blood pressure and to the variation of the artery diameter, which depends on the elasticity of the artery wall. Finally, PWV is proportional to the square root of the variation in blood pressure over that of the diameter and it depends on blood density.
C is the arterial compliance, PWV is the pulse wave velocity, E is the elasticity modulus, h is the thickness of the arterial wall, D is the arterial internal end-diastolic diameter and p is the density of the blood. dD the variation of the arterial diameter, and dP the blood pressure variation.
The measurement of PWV is of growing interest in this century. This concerns clinicians, physiologists, and engineers. Mathematical modeling of a tube with inhomogeneous liquid inside is very hard, especially in the case of a fluid with outstanding particle, pulsate and non-compressible flow, and propagating in elastic conduits.
Most of the modeling methods were described in the nineteenth and twentieth centuries. In the last decade, this subject has interested many Clinicians (O. Rourke, Safar, Lawrence, and Martyn). The direct measurement of arterial elasticity by the ratio between pressure and diameter using PWV was reviewed by Hickler in 1990, and Lehman Hayashi in 1999 and in 2002. Since then, there has been growing interest in non-invasive methods to measure AS, which reflects arteries compliance, using advanced technology to measure and signal processing of biological pulses.
9. The Carotid-Femoral Pulse Wave Velocity
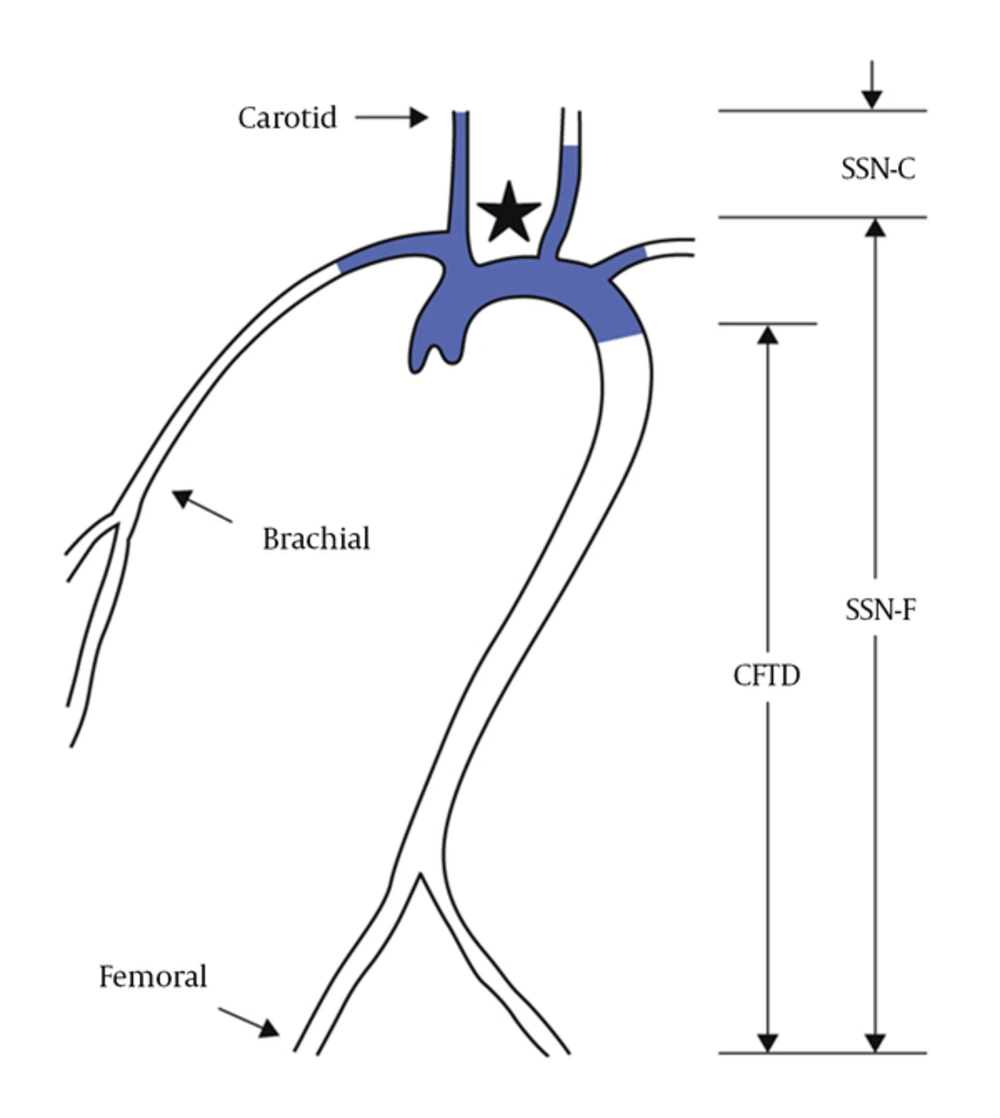
Several methods have been developed to noninvasively measure arterial compliance. Most of them are based on the determination of the carotid to femoral pulse wave velocity (PWVcf). The PWV between the common carotid artery (CCA) and the common femoral artery (PWVcf) is measured directly and corresponds to a well-accepted propagative model of the arterial system (14). Because it includes the aortic and aorto-iliac pathway, it is clinically relevant, because the big thoracic arteries (aorta and its first branches) represent the hemodynamic load that the left ventricle “sees” and are therefore responsible for a large part of the pathophysiological influence of arterial stiffness. Most epidemiologic studies demonstrating the predictive value of aortic stiffness for CV events have used carotid-femoral PWV. The CfPWV is considered as the gold standard for measuring arterial stiffness (13). In contrast, PWV measured outside the aortic track, for instance on the upper (brachial-radial PWV) or lower limb (femoral-tibial PWV), does not provide any additional predictive value for patients with end-stage renal disease. The pulse wave is generated by the contraction of the left ventricle, which causes initial shock pulse, counter the blood in the aorta. The pressure wave is recorded at these 2 sites (carotid and femoral) using sensors alternately on the carotid and the femoral site (42). Figure 4 shows the locations and the distance between the carotid and the femoral sites. To date, the PWVcf has been the best surrogate of vascular stiffness. It is now considered as a major point in cardiovascular risk management (4).
The conventional method for measuring direct PWV, using Pulsed Doppler sensor, seems tedious and expensive. Conventional methods use pressure sensors (piezoelectric, Complior®, Alam medical France; SphygmoCor®, Atcor, Australia; PulsPen®, Diatecne, Italy) laid in 2 points of an arterial segment. They detect the transit time between 2 points; the velocity of the pulse wave is defined as the ratio of the distance (D) between the 2 probes divided by the wave transit time (dt) (Figure 2). The distance is measured on the body surface between the carotid and the femoral sites. This method does not perfectly reflect the reality of the length of the arterial path, yet, it is considered as the gold standard. Controversies exist on how to measure this distance, given the carotid bifurcation (Figure 4). These methods overestimate the aortic path in obese subjects.
10. Indirect Methods
Other AS assessment methods use transfer functions. These techniques rely on simplified circulation models and are used when a single site for measuring the pressure waveform is required. They primarily measure the blood pressure at the arm using an ordinary device. The augmentation index and the central blood pressure are calculated according to age, blood pressure, and the morphology of the pulse wave. These methods and indices do not provide any additional predictive value beyond that of blood pressure measurement (43).
Devices close to the reference method apparatus (Omron®, Collin, Japan) use the pulse wave velocity and deduce the compliance using arms and legs cuffs. Brachial Ankle PWV (PWVba) measures arterial stiffness indices. The difference time (Dt) between the two fronts of the brachial and ankle pulse waves are taken into account. The direct distance from the arm to the ankle is measured, and is divided by dt, to obtain the PWVba.
The QKD is a concept that measures compliance regional outpatient (Diasis®, Novacor, France). Other devices, such as Tensioclinic Arterigraph® (Hungary), Mobilograph, are based on the enhancement of the reflected wave signal on an oscillometric plot when supra-systolic occlusion is obtained by a cuff on the arm. These “one-point measurements” of a velocity, use transfer functions, and they are called stiffness indices, yet, they do not have the value of 2 points of measurement of the aortic pulse wave velocity.
Physioflow® is a method based on impedance probes and they measure the flow to estimate the compliance of the aorta. Weinmann and Sapuznikov (44) have described a method that measures the pulse rate using photodiodes that allows the detection of pulse wave. This method is considered reproducible. The waveform sensed in fingertips has the same characteristics as the radial wave.
Finally, a recently developed is pOpmetre® (Axelife SAS, France). It is based on the assumption that the arteries of the upper limbs are of the same constitution of that of the lower limbs. They are muscular conducting arteries and not elastic. The lack of stiffening through aging of these arteries has been demonstrated. The pOpmetre® is also based on measuring the transit time of the pulse wave between the finger and the toe pulpar arteries (45) using photodiode sensors. The data provided by this apparatus are linked to aging (46), and has excellent intersession repeatability (SD/Mean = 5.7%) and a good correlation with the standard method. The algorithm was reviewed and the data of 101 subjects was used to compare pOpmetre® and the carotid to femoral gold standard method, and the 2 methods were very highly correlated (r = 0.91); mean ± SD 9.6 ± 1.7 vs 9.7 ± 1.6 (47); the bias between the 2 methods was 0.3 m/s using Bland and Altmann diagram. These results suggest that pOpmetre® PWV is a very robust surrogate for the gold standard carotid to femoral PWV. Other data were obtained in different pathologies with pOpmetre: in sickle cell disease (48), link to anti-phospholipids syndrome (49); pOpmetre PWV was associated to blood concentration of the Ac-APL. In systemic sclerosis PWV was higher in comparison to controls (50). The PWV with pOpmetre increased from control, obese, patients with diabetes, and obese-diabetic patients (51). It was linked to carotid plaques in metabolic syndrome (52) while Ankle Brachial Index was not different. The PWV with pOpmetre was liked to the Glomerular Filtration Rate in kidney function of transplants (53). Arterial Stiffness has been studied in many pathological populations for cardiovascular risk assessment, such as in patients with diabetes (54) and kidney transplant recipients (55). There is more than 2000 papers every year dealing with Pulse Wave velocity. pOpmetre was studied in a cardiac rehabilitation program and showed a link between the 6-minute walk test, PWV and maximal workload (MWL) that increased from 94.9 ± 35 to 116 ± 37 Watts and the 6-minute walking test (6MWT) from 430 ± 113 to 505 ± 106 m (P < 0.0001). The PWV decreased from 9.16 ± 3.0 to 8.39 ± 2.5 m/s (P < 0.008). A positive correlation was found with age (r = 0.38; P < 0.0003) and inverse correlation with maximal workload (r = -0.34; P < 0.001) and 6MWT (r = -0.22; P < 0.003) (56), as shown by Khoshdel et al. (57) in the link between kidney failure and exercise. Finally, at a medical health care center (22) they found that the PWV data in healthy individuals with pOpmetre® (N = 700) was exactly overlapping with that of 1500 cfPWV. While the carotid-femoral methods have a repeatability of around 12.3% to 14.5% (58), this latter method seems feasible in every day practice in outpatient clinics.
Nevertheless, the carotid to femoral PWV is used today in research centers, and the most important difference between these methods is that pOpmetre users need 2 hours of training to get a result within 20 seconds while 3 weeks is required to learn the carotid to femoral PWV and 15 minutes is needed to obtain results. With pOpmetre, there is no need to undress the patient for the femoral artery access and seems to be stress less and more acceptable for cultural reasons.
11. Conclusions
It has been shown that the measurement of vascular aging with PWV is a decisive biomarker for the early detection and management of at risk patients. Studies show the difficulties of implementation of the PWVcf. Indeed, 20% of cases tested for PWVcf are not measurable (59), and from personal experience, PWVcf was possible only in 80 people aged 70 and over among 120 tested patients because of stiff neck or the thickness of the abdominal apron. In the elderly, as in young adults, AS is a parameter to be taken into account in cardiovascular evaluation and general screening. It should be taken into account in various diseases specific to aging, such as osteoporosis and cognitive disorders (60, 61). Measuring vascular aging has been made simple, easy, and reliable, given its importance in several common diseases. This is recommended on a bi-annual basis for monitoring and management of vascular risk, as approved by the European and French societies of hypertension, and that of cardiology. The link between coronary artery stenosis is at the same level of significance as that of coronary arteries scoring (62). It adds 10% to 20% to the coronary heart disease risk score (63) and can be used in primary care screening for cardiovascular risk.
Noninvasive measurement was permitted at the end of the twentieth century and early twenty-first century, thanks to improved techniques capturing and signal processing to obtain the pulse signal with current technologies. pOpmetre® is a user-friendly equipment, simple and fast, with a short learning curve and reliable enough to measure arterial aging, suitable for people of all ages, in mass screening to better prevent, and manage cardiovascular therapy.