1. Background
Abortion means the loss of a pregnancy product (fetus or embryo) before the 20th week of pregnancy (1). Abortion and preterm childbirth in pregnant women may be due to inflammation caused by a febrile illness (2, 3); in this regard, Brucella is one of the infections whose role in pregnancy outcome has been discussed (4).
Brucellosis infection induces a TH1-type immune response. Enhancing this type of immune response is associated with an increase in factors such as gamma-interferon, etc. (5), which surveying the development of animal models of pregnancy, especially the mouse model, revealed its key role in abortion cases following Brucella infection (5).
It seems that the amount of gamma-interferon in the placental replacement phase is the most important factor in implantation, and the slightest increase in this amount can cause abortion (6). Additional research has shown that gamma-interferon performs this process by activating T lymphocytes and secreting cytokines recently used to induce abortion due to their effects on trophoblasts (7).
The presence of anti-Brucella antibodies in amniotic fluid has also reduced Brucella’s role in causing complications during pregnancy (3). However, in endemic areas, pregnancy outcomes in humans with brucellosis are similar to infected animals so that from normal childbirth to abortion, intrauterine fetal death, preterm childbirth, and placental abruption associated with brucellosis have been reported in pregnant women (4, 8-10).
During pregnancy, the disease can range from mild to severe and cause fever, intrauterine bleeding, and fetal membrane rupture, which are associated with miscarriage and sometimes stillbirth (6, 11, 12). These factors are important because of the endotoxin activity of this bacterium and the production of interleukin-1, as well as the effect on uterine smooth muscle, which all play an important role in inducing abortion (13). Generally, the highest rate of abortion occurs in the first and second trimesters of pregnancy, and the most obvious symptoms during this period are uterine bleeding and fever (14, 15). In these cases, rapid antibiotic treatment has been shown to have good effects in preventing abortion (16).
Therefore, paying attention to this issue is essential in the prompt and timely treatment of pregnant women with brucellosis; however, educating women of childbearing age, especially those of lower social and economic classes, regarding brucellosis can help prevent the disease and its complications during pregnancy. As mentioned above, the definitive diagnosis of Brucella is made by finding organisms in blood, body fluids, and tissue samples. At the same time, diagnosis of this disease is possible based on locality, clinical signs, standard serological tests, and blood culture (17, 18). However, it seems that due to the lack of a regular brucellosis screening program in periodic pregnancy examinations in endemic areas, many cases of brucellosis during pregnancy are not diagnosed or misdiagnosed (19).
Brucellosis is found all over the world, especially in the countries located in the Mediterranean area (Southern Europe and North and East Africa), the Middle East, India, and Central Asia (20, 21). In Iran, this disease is known as an endemic, occurring with different clinical forms and different prevalence models (4), and in the report of the disease spread in the country in 2010, Kerman province was reported as one of the regions with a moderate infection level (22). Thus, in Kerman province, surveying brucellosis infection regarding possible effects on pregnancy and abortion is particularly important.
2. Objectives
Since blood culture is considered a standard diagnostic method for detecting the Brucella microorganism in blood samples and has nevertheless received less attention in previous studies, the present study aimed to measure and compare cultivated blood and serum levels of Brucella antibodies between pregnancies leading to abortions and successful pregnancies regarding traceability of Brucella microorganisms in blood samples and their effects on pregnancies leading to abortions or successful pregnancies.
3. Methods
The present case-control study was conducted to compare blood culture and serum levels of anti-Brucella antibodies between pregnancies leading to abortion and full-term pregnancies in Afzalipour Medical Educational Hospital affiliated with Kerman University of Medical Sciences in 2020.
Women with spontaneous abortions without a clear cause referring to the hospital were considered a case group. The control group, consisting of women with normal pregnancy outcomes, visited the gynecology and obstetrics clinic for prenatal care. The sample size was determined to be equal to 34 people in both case and control groups, but in order to increase the validity, reliability, and generalizability of the findings, the number of samples was increased to 60 people in each group. Also, analytical and statistical tests were used to compare the mean scores between the two groups to ensure that the control group matched exactly with the case group. In all stages of the study, the P-value < 0.05 was accepted as a significant level.
At the beginning, the study objectives were explained to the participants, and after receiving all their answers, verbal informed consent was obtained from the participants to participate in the study. The individuals who refused to give a blood sample for testing, whose gestational age was unknown, and who had a specific reason for having an abortion were excluded.
Then, a self-made questionnaire whose validity was confirmed by experts and whose reliability was confirmed using Cronbach's alpha coefficient (0.7) was completed by face-to-face interview.
This questionnaire included demographic information for both groups, gestational and maternal age, the number of pregnancies, the number of previous abortions, a history of clinical or laboratory symptoms of Brucella, a recent history of contact with the livestock or their products, and a history of consuming unpasteurized dairy products consumption.
The researchers first sterilized 10 cc of each person's blood sample from a separate vein not attached to the branol. Then, 7 cc of Castaneda was added to the culture medium, which is two-phase (after disinfecting the culture medium door). At the same time, 3 cc of blood sample was injected into the clot tube for serological testing, and both were prepared for serology and culture.
In the laboratory, the culture medium was incubated vertically in a 37°C incubator, and after 48 hours, the medium was examined every two days; if it did not grow in the solid part of the colony, we would shake the glass and bring the liquid medium to the solid so that if there were bacteria in the blood, it would reach the solid surface and produce a colony.
In the laboratory, on days 14 and 21, after incubation for testing the Brucella subtypes, the plates were placed in Brucella agar and blood agar culture medium, and they were also placed in the presence of CO2 in the 37°C incubator for 48 hours. If the cultures were positive, a spread of it was hot stained and placed under a microscope for direct observation of the microorganism, and if the result was negative, the final result was reported.
Then, for serological testing, IgM and IgG levels (with 93.2% sensitivity and 99.3% specificity), which were measured using IBL Hamburg factory kits, the sample in the clot tube was stored in the freezer at -20°C after centrifugation and separation of serum until the serological testing to determine IgM and IgG serum levels, and finally, after collecting the required amount, samples were examined for serological testing. After completing the laboratory work, the obtained data were entered into SPSS software version 20 for statistical analysis according to the project objectives, and descriptive and analytical t-tests were used between different data groups.
4. Results
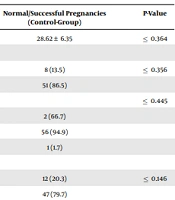
Of 120 participants, 60 were in the case group, and 60 were in the control group. The mean age of mothers in the case and control groups was 29.66 and 28.62, respectively. The mean age, the frequency of living in endemic areas of the province, a history of clinical symptoms and brucellosis, a history of contact with livestock and non-pasteurized local dairy products consumption, the mean gestational age, and the number of pregnancies and abortions showed no significant differences between case and control groups (Table 1).
| Demographic Variables | Pregnancies Leading to Abortions (Case-Group) | Normal/Successful Pregnancies (Control-Group) | P-Value |
|---|---|---|---|
| Maternal age | 29.66 ± 5.19 | 28.62 ± 6.35 | ≤ 0.364 |
| The place of residency | |||
| Endemic | 4 (8.3) | 8 (13.5) | ≤ 0.356 |
| Non-endemic | 44 (91.7) | 51 (86.5) | |
| The history of brucellosis | ≤ 0.445 | ||
| Yes | 1 (2) | 2 (66.7) | |
| No | 45 (91.8) | 56 (94.9) | |
| No idea | 3 (6.1) | 1 (1.7) | |
| The history of contact with livestock | |||
| Yes | 16 (32.7) | 12 (20.3) | ≤ 0.146 |
| No | 33 (67.3) | 47 (79.7) | |
| The history of unpasteurized dairy consumption | |||
| Yes | 30 (50.8) | 29 (49.2) | ≤ 0.210 |
| No | 19 (38.8) | 30 (50.8) | |
| The gestational age | |||
| Weeks of pregnancy | 11.65 ± 3.77 | 38.25 ± 1.25 | ≤ 0.001 |
| The number of pregnancies | |||
| Number of pregnancies | 3.14 ± 0.17 | 2.38 ± 0.14 | ≤ 0.001 |
| The number of abortions | |||
| Number of abortions | 1.38 ± 0.63 | 0.30 ± 0.06 | ≤ 0.001 |
Abbreviation: SD, standard deviation.
a Values are expressed as mean ± SD or No. (%).
The results showed that 3.33% of the participants in the case group (2 people) had a positive titer of IgM, and comparing the number of participants between case and control groups for a positive IgM titer showed no significant differences (P ≤ 0.058) (Table 2).
| Antibody Titer | Pregnancies Leading to Abortions (Case-Group) | Normal/Successful Pregnancies (Control-Group) | Total | P-Value |
|---|---|---|---|---|
| Positive | 2 (3.33) | 0 (0.0) | 2 | ≤ 0.058 |
| Borderline | 11 (18.34) | 3 (5) | 14 | |
| Negative | 47 (78.34) | 47 (78.34) | 94 | |
| Hemolysis | 0 (0.0) | 10 (16.7) | 10 | |
| Total | 60 (100) | 60 (100) | 120 |
Although 3.33% of the participants in the case group (2 people) had a positive titer of IgM, no positive titer of IgM was found among the controls, and the comparison of the number of individuals between the two groups for a positive IgG titer showed no significant differences either (P ≤ 0.206) (Table 3).
| Antibody Titer | Pregnancies Leading to Abortions (Case-Group) | Normal/Successful Pregnancies (Control-Group) | Total | P-Value |
|---|---|---|---|---|
| Positive | 2 (3.33) | 0 (0.0) | 2 | ≤ 0.206 |
| Borderline | 4 (6.67) | 1 (1.66) | 5 | |
| Negative | 54 (90.00) | 49 (81.67) | 103 | |
| Hemolysis | 0 (0.0) | 10 (16.7) | 10 | |
| Total | 60 (100) | 60 (100) | 120 |
The results of the analytical t-test showed that the mean IgM level in the case and control groups was 5.66 ± 0.36 and 4.75 ± 0.23, respectively, and the difference between the two groups was significant (P ≤ 0.042).
The mean IgG serum level in the case and control groups was 3.33 ± 0.32 and 2.92 ± 0.18, respectively, showing a significantly higher amount in those with an abortion (P ≤ 0.300) (Table 4).
| Antibody Serum Level | Pregnancies Leading to Abortions (Case-Group) | Normal/Successful Pregnancies (Control-Group) | P-Value |
|---|---|---|---|
| IgG | 3.33 ± 0.32 | 2.92 ± 0.18 | ≤ 0.300 |
| IgM | 5.66 ± 0.36 | 4.75 ± 0.23 | ≤ 0.042 |
Abbreviation: SD, standard deviation.
a Values are expressed as mean ± SD.
Although 3.33% of participants in the case group had positive blood cultures, no positive blood culture was observed in the control group, and no significant difference was observed between the two groups regarding positive blood cultures (P ≤ 0.157) (Table 5).
| Brucella Detected in Blood Culture | Pregnancies Leading to Abortions (Case-Group) | Normal/Successful Pregnancies (Control-Group) | Total | P-Value |
|---|---|---|---|---|
| Positive | 2 (3.3) | 0.0 | 2 (1.7) | ≤ 0.157 |
| Negative | 58 (96.7) | 60 (100) | 118 (98.3) |
The mean IgM level in the group with abortions under 12 weeks was 4.97 ± 0.43, and the mean IgM level in abortions over 12 weeks was 6.67 ± 0.68, which was significantly different from abortions in the first trimester (P ≤ 0.043) (Table 6).
| Antibody Serum Level | Abortions Less Than 12 Weeks | Abortions More Than 12 Weeks | P-Value |
|---|---|---|---|
| IgG | 3.56 ± 0.47 | 3.02 ± 0.38 | 0.422 |
| IgM | 4.97 ± 0.43 | 6.67 ± 0.68 | 0.043 |
Abbreviation: SD, standard deviation.
a Values are expressed as mean ± SD.
5. Discussion
The present study showed no significant relationship between IgM and IgG-positive titer among participants with successful pregnancies and abortion, while today, investigating the consequences of Brucella infection during pregnancy is one of the complex issues of infectious diseases receiving much attention due to the uncertainty in Brucella's ability in playing an explained role in pregnant women.
This problem in endemic areas, where the complications take a more cohesive form and occur as a regular pattern of abortion, fetal death in utero, and preterm childbirth, has led some researchers to suggest prophylactic treatments for pregnant women in such areas (23).
In a study conducted by researchers at the Razi Institute in Iran, Brucella melitensis was isolated from the remaining tissues of the placenta and aborted human fetus in two cases, and the theory was raised that brucellosis could cause abortion in the second trimester of pregnancy. Several studies have also reported the possibility of abortion, mostly in the second trimester (24), which is comparable to our clinical observations about the rate of abortions in the first and second trimesters of pregnancy.
The results of another study by Khan et al. regarding brucellosis in Saudi Arabia on 545 pregnant women, of whom 92 were found to have brucellosis, showed that in an endemic area, spontaneous abortion and the rate of intrauterine fetus death caused by brucellosis were 43% and 2% respectively. All of these patients had antibody titers above 1.320, but the mean titer was reported to be above 1.2560. Despite the high antibody titer and rate of abortion, no significant relationship was found between the titer and the incidence of abortion (15). Similar to our findings, comparing patients for positive IgG titers revealed no significant differences between case and control groups. In contrast, 3.3% (2 people) in the case group had a positive titer of IgG, and no positive IgG was found in the control group.
In Roushan et al.’s study on 19 pregnant women with brucellosis, of whom 10 (53%) had a miscarriage in the first trimester of pregnancy, the researchers were able to give birth to 9 healthy full-term infants with a combination of antibiotic therapy, including rifampin plus cotrimoxazole on 13 patients, and only 4 (13%) mothers had miscarriage despite treatment (8); the findings of the mentioned research emphasize the role of antibiotic treatment in prevention of adverse consequences in case of brucellosis. Our clinical observations also showed fever and uterine bleeding, similar to the most obvious symptoms, which can indicate the need to use antibiotic treatments.
Turkey is another country that has received numerous reports of brucellosis due to its endemicity. For example, Kurdoglu et al. evaluated the effects of brucellosis on pregnancy in 29 pregnant women with brucellosis. At the same time, by the enzyme-linked immuno-sorbent assay (ELISA) method, the roles of other microorganisms, such as herpes virus, cytomegalovirus, rubella virus, and toxoplasma parasite, were ruled out, and the patients were then followed up. Among all patients, 7 (24.11%) cases had a miscarriage, one (3.45%) case had intrauterine death, and two (6.9%) cases had preterm childbirth, and the researchers were able to isolate Brucella bacterium from the blood cultures of only two patients. The noteworthy point was the childbirth of a healthy full-term infant in 19 (65.5%) patients (20). At the same time, a study conducted in Turkey (10) pointed to the importance of taking the management and treatment of the disease seriously in endemic countries like our country. Nassaji et al. investigated the relationship between asymptomatic Brucella infection and miscarriage in 103 women with normal pregnancy outcomes as a control and 81 women with spontaneous abortions as a case group with IgG and IgM antibodies measurement using the ELISA method and found no significant relationship between brucellosis and abortion regarding antibody levels (25), which is compatible with part of our results. On the contrary, Vilchez et al. revealed that the rate of miscarriage in pregnant women infected by Brucella with an antibody titer higher than 1.160 increased significantly, suggesting that active bacterial infection could be a potential risk factor for spontaneous abortion (26). As mentioned above, our findings also showed a positive titer of IgG in the case group, which may suggest the use of antibody titer for the detection of Brucella infection.
Different studies have shown various results. For example, Kurdoglu’s research showed that 10 out of 27 pregnant mothers with brucellosis died, the results of which were not significant (27). Also, Gulsun et al.’s study showed no significant level of abortion among women with brucellosis and normal women (10).
Generally, in most cases, the highest pregnancy loss rate occurred in the first and second trimesters, while according to other studies, the most obvious symptoms during this period were uterine bleeding and fever (15, 27); these findings are the same as our results.
Also, as previously mentioned in a study by researchers at the Razi Institute in Iran, Brucella melitensis was isolated from the remaining tissues of the placenta and aborted human fetus in two cases, suggesting that brucellosis could cause abortion in the second trimester of pregnancy (18). In contrast, in these cases, rapid antibiotic treatment has been shown to have good effects in preventing abortion, which is also in line with another study in Turkey (10).
Different results were obtained from studies conducted with similar purposes. For example, in Sharif et al. and Elshamy and Ahmed's study, a significant relationship was found between serum levels of individuals with successful pregnancies and those with abortion (2, 14, 26), compared to our results showing a significantly higher mean serum level of IgG in the case group and no significant relationship between both case and control groups regarding the mean serum level of IgM. In other studies, no significant association was found either (8, 15, 19). A high antibody titer usually (but not always) indicates an active infection in the body, and according to all the above-mentioned materials, brucellosis is likely to increase the risk of miscarriage or premature birth.
However, in our findings, the mean level of IgG was significant between case and control groups, with a higher significant amount in the case group. Another study by Staalsoe et al. found high IgM levels in pregnant women; however, this amount has no effect on pregnancy outcomes and can only affect the quality of the health of newborns (28).
The impact of brucellosis in pregnancy is still unresolved worldwide and needs further investigation. However, according to this study, there is no difference between positive blood culture and serum levels of anti-Brucella antibodies in pregnancies leading to abortion and successful pregnancies, but regarding different studies reporting various results from endemic countries, what is clear is that antibiotic treatments should be started at the first place for pregnant women with brucellosis, which is the most effective way to treat the disease and prevent consequences. Also, developing educational strategies for women of childbearing age, especially those of lower social and economic classes, will help prevent the disease and its adverse complications in pregnancy.
Due to the higher mean IgM level in the case group and the lower laboratory threshold level, an increase in the study population, and the evaluation of other complications of Brucella on pregnancy outcome (intrauterine death, preterm delivery, low birth weight, etc.), sampling in the endemic areas or conducting a study on people who have clinically and laboratory-confirmed Brucella infection before entering the study can provide more accurate results of the brucellosis effects on the outcome of pregnancy in humans. Although this study was conducted in the largest gynecology and obstetrics center of Kerman province, which is considered a referral center for southeastern Iran and a large endemic area for brucellosis, the effects of Brucella on pregnancy outcomes should be more accurately estimated by providing laboratory and necessary facilities for keeping samples and using a larger sample size.