1. Background
Fear and anxiety can be natural responses to specific situations and stressful events (1, 2). However, panic disorder differs from natural fear and anxiety, as it is often intense and may seem abnormal (3). It is classified as a chronic, periodic, and disabling anxiety disorder (4). Panic disorder is characterized by sudden episodes of intense fear and recurrent panic attacks (5). These attacks typically do not last more than a few minutes and are accompanied by symptoms such as difficulty breathing, chest pain, pounding or racing heart, sweating, shortness of breath, nausea, and feeling faint or dizzy (6). The intensity of these symptoms can lead patients to believe they are having a heart attack and fear that they are going to die (7).
Rumination refers to overthinking that becomes highly distressing and painful (8). The mind of a person with panic disorder is often preoccupied with negative and upsetting thoughts, which contributes to the persistence of symptoms (9). Therefore, rumination is a psychological factor that plays a key role in panic disorder (10). It is defined as periodic, persistent anxious thinking, which is a relatively common response to negative moods and a prominent cognitive characteristic of feelings of blame and panic disorder (11).
Self-inhibition can mitigate the symptoms of patients with panic disorder. Inefficient inhibitory processes can profoundly affect life and induce impulsive behaviors, which are generally harmful (12). As a metacognitive concept, self-inhibition can be defined as the voluntary, conscious, and effortful control of attention, thoughts, feelings, and behavior (13). Self-inhibition and self-control are effective strategies for mental health; moreover, emotional and behavioral adjustment disorders are associated with a wide range of psychological conditions, including anxiety, depression, drug abuse, and engaging in aggressive behaviors (14).
In general, panic disorder strongly affects the quality of life (15). The concurrent prevalence of other psychological and medical disorders in individuals with panic disorder requires additional attention (16). Researchers have used transcranial direct-current stimulation (tDCS) to improve various disorders (17-19). This method has been employed over the past two decades to enhance motor and cognitive functions and to treat neurological and psychological disorders (20). During the tDCS process, a mild current passes through the skin and cranium to neural tissues, altering cortical excitability. Common tDCS protocols involve cortical stimulation using a direct current with two interconnected electrodes on the skin: One anode and one cathode. A 1-mA or 2-mA current is applied between these electrodes for 20 minutes, with each electrode having a cross-sectional area of 35 cm² (21). The current flows from the anode to the cathode, and its direction and intensity up-regulate and down-regulate cortical excitability (22). Research has shown that tDCS is effective in alleviating rumination and depression symptoms (19, 23), improving response inhibition in patients with obsessive-compulsive disorder (OCD) (24), mitigating rumination, automatic negative thoughts, and psychological symptoms (e.g., anxiety and depression) in patients with major depressive disorder (25), decreasing cravings for drug use and enhancing cognitive self-control in drug abusers (26), and improving depression, anxiety, and rumination in patients with posttraumatic stress disorder (27).
Given the significant prevalence of panic disorder and the various associated challenges, psychologists and psychotherapists have been seeking different effective treatments for this condition. However, the effects of tDCS on the psychological problems of patients with panic disorder have not yet been evaluated.
2. Objectives
Therefore, based on these considerations, the present study aimed to investigate the effectiveness of tDCS on rumination and self-inhibition in patients with panic disorder.
3. Methods
The statistical population included all patients with panic disorder visiting psychological centers in Ahvaz, Khuzestan Province (Iran), in 2023. Convenience sampling was used to select 40 eligible patients, who were then randomly assigned to an experimental group (n = 20) and a control group (n = 20). According to Gall (28), experimental and quasi-experimental studies typically include 15 - 20 participants in each group (i.e., experimental and control). The inclusion criteria were being diagnosed with panic disorder by a therapist, providing conscious consent, having at least a middle school education, being aged 18 - 45, scoring above average on the rumination questionnaire and below average on the self-inhibition questionnaire, having no history of drug abuse, participating in no concurrent therapies, and receiving no individual consultation or pharmaceutical treatment. The exclusion criteria included receiving concurrent psychotherapeutic treatments, using psychiatric drugs, unwillingness to continue participation in the research, encountering a highly stressful event, and failing to attend more than two therapeutic sessions. After the pretest stage, the experimental group underwent the treatment, while the control group received no intervention. Following the completion of the treatment, the posttest stage was conducted for both groups. The treatment was also provided to the control group in a super-intensive format after the research was completed.
3.1. Tools
3.1.1. Ruminative Response Scale
Developed by Nolen-Hoeksema et al. (29), the Ruminative Response Scale (RRS) measures responses to negative moods. It consists of two subscales: “Reflective pondering” and “brooding,” each with eleven items. The 22 items of the RRS are scored on a 4-point Likert scale ranging from 1 (never) to 4 (often). The minimum and maximum scores are 22 and 88, respectively. In Mousavi et al.'s research (8), Cronbach's alpha was 0.90.
3.1.2. Self-restraint Scale
Developed by Weinberger and Schwartz (30), the Self-restraint Scale (SRS) comprises four subscales and consists of thirty items scored on a 5-point Likert scale. The subscales are suppression of aggression, impulse control, consideration of others, and responsibility. Scores of 1, 2, 3, 4, and 5 correspond to “always,” “often,” “sometimes,” “rarely,” and “never,” respectively. The SRS has a maximum score of 150 and a minimum score of 30, representing the highest and lowest levels of emotional inhibition, respectively. In Roustaei et al.'s research, the retest reliability for the SRS was reported to be 0.89 (31).
3.2. Intervention
The tDCS Device: The experimental group received ten 20-minute tDCS sessions. This study used a Neurostim tDCS device (MedinaTeb Co., Iran), provided by the Sina Cognitive Science Institution. Introduced to the market in 2015, the device can deliver five types of stimuli. It has two separate channels, each of which can be independently adjusted to apply a specific stimulus. Several stimulation parameters, such as current, time, and frequency, can be adjusted on this device. The Neurostim tDCS device is equipped with a rechargeable battery, providing an effective operating time of up to eight hours. Sponge pads, measuring 5.3 × 5.3 cm, were used on the electrodes, and a saline solution was applied to wet the pads.
3.3. Data Analysis
Analysis of covariance (ANCOVA) was used for data analysis in SPSS 27.
4. Results
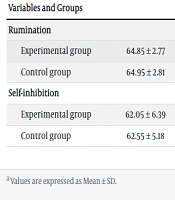
Regarding demographics, 44% of the participants were aged 18 - 32 years, while the remaining 56% were aged 33 - 45 years. The average age was 27.30 in the experimental group and 31.40 in the control group. The mean, standard deviation (SD), and Kolmogorov–Smirnov test results for rumination and self-inhibition are presented in Table 1.
| Variables and Groups | Pretest | Posttest | Kolmogorov–Smirnov | |
|---|---|---|---|---|
| Z | P | |||
| Rumination | ||||
| Experimental group | 64.85 ± 2.77 | 40.00 ± 5.65 | 0.18 | 0.089 |
| Control group | 64.95 ± 2.81 | 65.25 ± 3.98 | 0.12 | 0.200 |
| Self-inhibition | ||||
| Experimental group | 62.05 ± 6.39 | 111.50 ± 7.50 | 0.17 | 0.092 |
| Control group | 62.55 ± 5.18 | 62.45 ± 6.10 | 0.14 | 0.200 |
Mean ± SD and Kolmogorov–Smirnov Test Results of Rumination and Self-inhibition in Experimental and Control Groups a
According to Table 1, the experimental group showed different means in the posttest stage compared to the pretest stage, whereas the means of the variables for the control group remained nearly unchanged. Therefore, ANCOVA was employed to evaluate the significance of the differences between the two groups. Before conducting the ANCOVA, the test assumptions were analyzed. The Kolmogorov–Smirnov test confirmed the absence of major outliers (Table 1), thus verifying the normality of the data distribution for ANCOVA. Levene's test was used to evaluate the homogeneity of variance, with the F-value and P-value reported as 0.647 and 0.426 for rumination, and 3.385 and 0.055 for self-inhibition, respectively. To compare the control group with the experimental group on posttest scores, the effects of the pretests were controlled.
ANCOVA was used to measure the effects of the tDCS intervention on rumination and self-inhibition in patients with panic disorder. Table 2 reports the posttest results. Based on the effect sizes, the tDCS intervention accounted for 88% of the variance in rumination and 94% of the variance in self-inhibition. The statistical exponent of the test was 1, indicating that the sample size was adequate. There was a significant difference between the pretest and posttest means for rumination after controlling for the pretest effect (F = 285.05, P < 0.001). This suggests that tDCS can be effective in alleviating rumination. Additionally, a significant difference was found between the pretest and posttest means for self-inhibition (F = 643.90, P < 0.001), indicating that tDCS can also be effective in improving self-inhibition in patients with panic disorder.
| Variables | SS | df | MS | F | P | η2 | Power |
|---|---|---|---|---|---|---|---|
| Rumination | 6328.18 | 1 | 6328.18 | 285.05 | 0.001 | 0.88 | 1.00 |
| Self-inhibition | 24142.74 | 1 | 24142.74 | 643.90 | 0.001 | 0.94 | 1.00 |
The Results of ANCOVA on Rumination and Self-inhibition in Experimental and Control Groups
5. Discussion
The present study aimed to investigate the effectiveness of tDCS on rumination and self-inhibition in patients with panic disorder. According to the findings, tDCS was effective in alleviating rumination in the posttest phase. This result is consistent with the study by Hoebeke et al. (23). In this regard, tDCS is a non-invasive technique designed to enhance cognitive and neurological functions through direct cortical stimulation with a small current (25). The effectiveness of tDCS in improving rumination depends on the stimulated area, as well as the duration and intensity of stimulation. Some studies have indicated that tDCS can improve memory, attention, data processing, and other cognitive functions (32, 33).
In the tDCS process, anodic current is used to up-regulate cortical excitability, while cathodic current is employed to down-regulate it. A small, continuous current flows through the head with electrodes positioned on the scalp (26). In other words, tDCS enhances excitability in targeted brain areas. The increased excitability in specific brain regions alters cognitive and behavioral functions and alleviates rumination in patients with panic disorder. Two networks are involved in rumination: The central executive network (CEN) and the default mode network (DMN). The dorsolateral prefrontal cortex (DLPFC) plays a crucial role in balancing the CEN and DMN, and thus optimizing thinking (34). Repeated stimulation of the DLPFC in both cerebral hemispheres is significantly effective in reducing rumination. Stimulation of the DLPFC helps alleviate rumination by modulating cognitive processing and negative emotional information in the right hemisphere and enhancing cognitive control over positive emotional stimuli in the left hemisphere.
The results also indicated that tDCS was effective in improving self-inhibition in patients with panic disorder in the posttest stage. While deep brain stimulation requires surgery, tDCS is applied to the brain through electrodes placed on the skin. Several studies have demonstrated that tDCS can significantly improve panic disorder symptoms, anxiety, and self-inhibition, particularly during the post-treatment period (17, 24). Direct current is delivered by a battery-powered DC generator, leading to long-term changes in cortical polarity after neuron depolarization and hyperpolarization, which can affect neural receptors. Unlike electrical shock treatment, tDCS is not ineffective and can substantially improve paranoid symptoms. It can also reduce levels of self-sufficiency in patients (19). Overall, tDCS is a neuro-based therapeutic technique that involves applying a small, direct current to the cortex, facilitating autonomous neural activities, and modulating brain functions to stimulate target areas. This technique uses a weak electric current to influence the brain and is employed to treat various psychological and neurological disorders.
This study faced certain limitations. It was conducted on patients with panic disorder in Ahvaz, Khuzestan Province (Iran), so caution should be exercised when generalizing the results to other populations.
5.1. Conclusions
According to the results, tDCS was effective in alleviating rumination and improving self-inhibition among patients with panic disorder. However, further research is needed to verify these results reliably. Overall, tDCS may be used as a complementary method for treating rumination and self-inhibition but should not be considered the primary treatment. Psychotherapists and psychological centers should consider tDCS for treating cognitive and behavioral disorders.