1. Background
Today, chronic and non-communicable diseases have severely threatened people's health due to lifestyle changes and various societal developments. Among them, four groups — cardiovascular diseases, cancer, respiratory diseases, and diabetes — pose the most critical threats to human life. The costs associated with their prevention, control, and treatment have imposed a heavy burden on families and healthcare systems (1).
Diabetes, recognized as the most common chronic non-communicable disease, is not just a single condition but a complex disorder influenced by both environmental and genetic factors. It is highly costly and disabling due to its association with other diseases, including cardiovascular complications, renal failure, vision impairment, and disability (2).
In diabetic patients, proper blood sugar control can prevent or delay the development of complications. People with diabetes are more susceptible to various complications and clinical manifestations, particularly affecting the heart, blood vessels, kidneys, nerves, eyes, and oral health. Oral manifestations of diabetes mellitus include periodontal disease (gum disease), which is more prevalent in diabetic individuals and progresses more rapidly than in non-diabetic individuals (3). Moreover, evidence suggests that periodontitis itself is a potential risk factor for diabetes mellitus (4).
Inflammatory gum diseases, caused by bacterial infections, are a form of chronic inflammation. Diabetic patients experience more severe and rapid gingival destruction due to microbial plaque formation in the mouth (5, 6). Gum abscesses are also more common among diabetics, primarily due to their reduced immune defense against infections (6). Additionally, these patients are more susceptible to various clinical forms of oral candidiasis compared to healthy individuals. Conditions such as zygomycosis and benign migratory glossitis may also occur in individuals with type 1 diabetes who do not maintain proper glycemic control. Diabetic sialadenosis, characterized by diffuse, painless bilateral swelling of the parotid glands, can be observed in both type 1 and type 2 diabetes (7).
Research on diabetes and its associated oral complications indicates that diabetic patients frequently experience cardiovascular, renal, neurological, eye, and oral health issues. Vascular problems, oxidative stress, and immune system imbalances create favorable conditions for the growth of various gram-positive and gram-negative bacteria, as well as different fungi, particularly Candida albicans, in the mouth and gums (8).
For patients with diabetes mellitus, especially type 1 diabetes, strict blood glucose control is crucial to reducing the frequency of disease-related complications. Meanwhile, for patients with type 2 diabetes, dietary modifications and increased physical activity are essential for disease management (9). The relationship between diabetes and oral health is not only bidirectional but also highlights diabetes mellitus as one of the most significant risk factors for oral and periodontal diseases (10).
Clinical studies have demonstrated that proper treatment of periodontitis can significantly contribute to improved blood sugar control in diabetic patients (11). Grossi and Genco reported substantial improvements in both periodontal and glycemic status following a combination of dental and antibiotic treatments (12). Additionally, a study evaluating the impact of a randomized periodontal intervention over an 18-month period found that the glycosylated hemoglobin (HbA1c) levels of patients in the intervention group improved significantly compared to the control group, placing them within the glycemic control range for diabetes (13).
2. Objectives
Given these findings, we designed this study to assess the incidence of periodontal disease in diabetic patients admitted to Imam Khomeini Hospital (RA), Iran.
3. Methods
This cross-sectional study was conducted in the Department of Internal Medicine at Imam Khomeini Hospital, Iran, from October 2022 to December 2022. The study population comprised known type 2 diabetic patients admitted to the endocrinology ward with glycemic disorders. All eligible participants were thoroughly informed about the nature, potential risks, and benefits of their participation in the study.
3.1. Inclusion Criteria
(1) Patients aged 30 - 65 years who have been diagnosed with type 2 diabetes for at least two years based on WHO criteria (14, 15) [random blood sugar (RBS) level ≥ 200 mg/dL, fasting plasma glucose ≥ 126 mg/dL, and 2-hour postprandial glucose ≥ 200 mg/dL].
(2) Patients with at least 20 teeth.
(3) Non-smokers and non-alcoholic patients.
3.2. Exclusion Criteria
(1) Pregnant and lactating patients.
(2) Patients using medications other than blood sugar control drugs.
(3) Patients who have received periodontal treatment within the last six months.
(4) Patients with autoimmune diseases.
(5) Patients with dental implants.
3.3. Sample Size
Based on recent evidence, the prevalence of periodontal diseases in patients with type 2 diabetes is reported to range between 25% and 98%. In the present study, considering the prevalence of periodontal diseases in Iran (4) and using a 5% margin of error (type I error: α = 0.05) and 80% power (type II error: 1-β = 0.80), a minimum sample size of 400 patients was determined using G Power software.
3.4. Methodology
In this cross-sectional study, conducted over three months (October 2022 to December 2022), 400 patients were examined. To evaluate awareness, attitude, and performance, a questionnaire containing 25 questions was used. Five questions assessed awareness, eight evaluated attitude, six measured performance, and the remaining questions gathered demographic information and data on the periodontal status of patients.
Patients' demographic information, including age, gender, disease duration, fasting blood sugar, HbA1c, BMI, and systolic and diastolic blood pressure, was recorded. The questionnaire was adopted from the study by Neville et al. (7), with a reliability coefficient of 0.72, and its content validity was qualitatively confirmed by experts.
The scoring system for awareness levels was categorized as weak (0 - 40), moderate (41 - 65), and good (66 - 100). Attitude levels were classified as weak (0 - 50), moderate (51 - 75), and good (76 - 100). Performance levels were rated as poor (0 - 30), moderate (31 - 70), and good (71 - 100). Patients completed the questionnaire in person, and if a patient was illiterate, the researcher read the questions aloud to them.
To assess periodontal health, oral symptoms such as dry mouth, unpleasant taste in the mouth, bad breath, loose teeth, swollen gums, sensitive teeth, gum bleeding, burning mouth and tongue, and a burning sensation in the gums were evaluated and recorded. Additionally, oral and dental hygiene compliance was assessed based on the use of a toothbrush, toothpick, dental floss, and mouthwash. Patients were also examined for chronic periodontitis, with severity categorized as mild, moderate, or severe.
3.5. Statistical Analysis
The findings were presented as mean ± standard deviation and frequency (percentage) using appropriate charts and tables. Student's t-test and Pearson correlation coefficient were used to assess the relationship between quantitative variables, while the chi-square test was employed to compare qualitative variables. A P-value of less than 0.05 was considered statistically significant. All analyses were conducted using SPSS software, Windows version 21.0 (Chicago, Inc., USA).
4. Results
4.1. Demographic Data
In this retrospective cross-sectional study, we evaluated 400 patients with diabetes at Imam Khomeini Hospital in Urmia, Iran. Among them, 214 patients (53.5%) were male, and 186 (46.5%) were female. The average age of the patients was 51.49 ± 5.73 years, with a minimum age of 37 years and a maximum of 61 years. Regarding education levels, 39 patients (9.75%) had education above a diploma, 294 patients (73.5%) had education below a diploma, and 67 patients (16.75%) were illiterate.
4.2. Patient's Diabetes Status
The average duration of the disease was 7.51 ± 3.12 years, with a minimum duration of two years and a maximum of 17 years. The findings related to the patients' diabetes are presented in Table 1.
| Variables | Result |
|---|---|
| Disease duration (y) | 7.51 ± 3.12 |
| Fasting blood sugar (mg/dL) | 198.69 ± 71.37 |
| HbA1c level (%) | 7.91 ± 0.49 |
| Diabetes control | |
| Normal (HbA1c < 6) | 41 (10.25) |
| Good (HbA1c = 6 - 7) | 87 (21.75) |
| Moderate (HbA1c = 7 - 8) | 94 (23.5) |
| Poor (HbA1c > 8) | 178 (44.5) |
| Blood pressure (mmHg) | |
| Systolic | 122.44 ± 0.29 |
| Diastolic | 82.49 ± 0.87 |
| BMI (kg/m2) | 25.39 ± 0.12 |
Abbreviation: HbA1c, glycosylated hemoglobin.
a Values are expressed as mean ± SD or No. (%).
4.3. Patient's Periodontal Disease Status
The oral symptoms of the patients are presented in Table 2. The prevalence of periodontitis among the studied patients was 88.25%. Regarding oral symptoms, the most common was dry mouth, occurring in 57.75% of patients, followed by an unpleasant taste in the mouth, reported by 22.25%. The least common symptom was a burning sensation in the gums, with a frequency of 11.25%.
| Variables | Result |
|---|---|
| Oral symptoms | |
| Dry mouth | 213 (57.75) |
| Bad taste in the mouth | 89 (22.25) |
| Bad breath | 69 (17.25) |
| Loose teeth | 87 (21.75) |
| Gum swelling | 66 (16.5) |
| Sensitive teeth | 67 (16.75) |
| Bleeding gums | 47 (11.75) |
| Burning mouth and tongue | 46 (11.5) |
| Burning in gums | 45 (11.25) |
| Severity of periodontitis | |
| No periodontitis | 47 (11.75) |
| Mild chronic periodontitis | 121 (30.25) |
| Moderate chronic periodontitis | 176 (44) |
| Severe chronic periodontitis | 56 (14) |
a Values are expressed as No. (%).
The frequency of patients' oral and dental hygiene status, along with their compliance with oral hygiene practices, is presented in Table 3. The majority of patients (72.25%) had an average oral hygiene status, and the most commonly used oral hygiene device was toothpicks (22%).
| Variables | Result |
|---|---|
| Tools used | |
| Toothbrush | 44 (11) |
| Toothpick | 88 (22) |
| Dental floss | 67 (16.75) |
| Mouthwash | 27 (6.75) |
| None | 174 (43.5) |
| Oral hygiene status | |
| Good | 92 (23) |
| Moderate | 289 (72.25) |
| Poor | 19 (4.75) |
a Values are expressed as No. (%).
4.4. Patients Awareness and Attitude Status
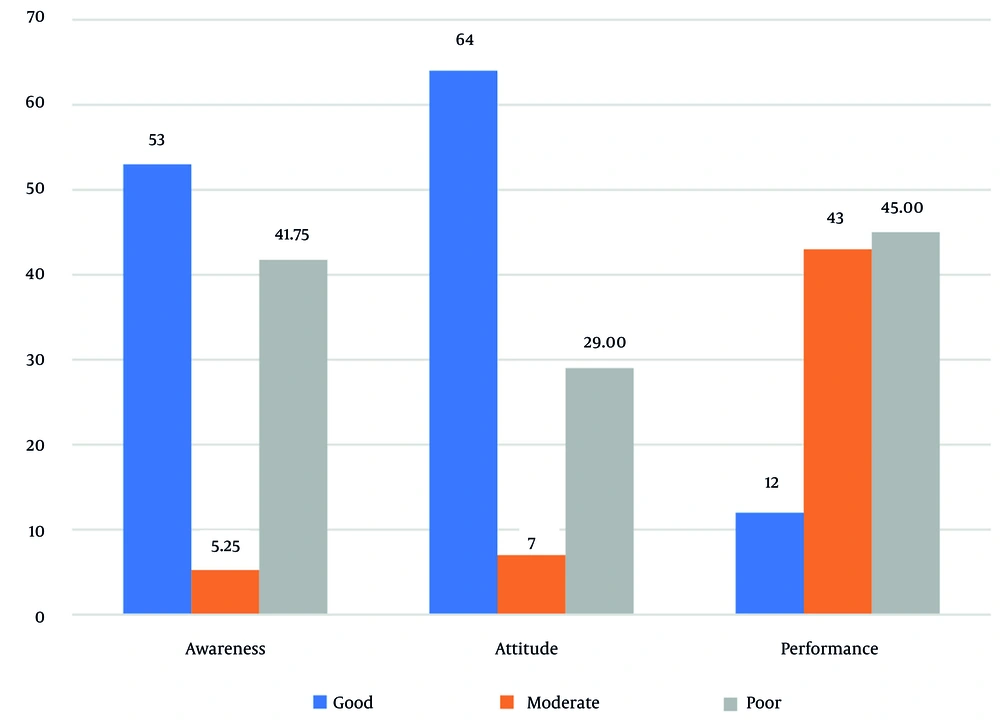
In assessing patients' knowledge, 53% demonstrated a good level of knowledge. Additionally, 64% of patients had a good attitude. However, in terms of performance, only 12% of patients exhibited a good level, while 45% had a poor performance level (Figure 1).
4.5. Diabetes and Periodontal Diseases Relations
The results of the analysis examining the relationship between the periodontal status of patients with diabetes, their diabetes control status, and their oral health status are presented in Table 4. The findings indicated a significant relationship between the periodontal status of diabetic patients and their diabetes control status (P = 0.001, χ2 = 87.19) as well as their oral health status (P = 0.001, χ2 = 101.17).
| Variables | Severity of Periodontitis | Total | |||
|---|---|---|---|---|---|
| Normal | Mild | Moderate | Severe | ||
| Total | 47 (11.75) | 121 (30.25) | 176 (44) | 56 (14) | |
| Diabetes control | |||||
| Normal (HbA1c < 6) | 29 (61.7) | 7 (5.79) | 3 (1.7) | 2 (3.57) | 41 (10.25) |
| Good (HbA1c = 6 - 7) | 4 (8.51) | 43 (35.54) | 30 (17.05) | 10 (10.86) | 87 (21.75) |
| Moderate (HbA1c = 7 - 8) | 11 (23.4) | 35 (28.93) | 47 (26.7) | 1 (1.79) | 94 (23.5) |
| Poor (HbA1c > 8) | 3 (6.38) | 36 (29.75) | 96 (54.55) | 43 (76.79) | 178 (44.5) |
| Oral hygiene status | |||||
| Good | 40 (85.1) | 6 (5) | 9 (5.1) | 37 (66.1) | 92 (23) |
| Moderate | 4 (8.5) | 108 (89.3) | 159 (90.3) | 18 (32.2) | 289 (72.25) |
| Poor | 3 (6.4) | 7 (5.7) | 8 (4.6) | 1 (1.7) | 19 (4.75) |
Abbreviation: HbA1c, glycosylated hemoglobin.
a Values are expressed as No. (%).
In evaluating the relationship between patients' age and the severity of periodontal disease, a direct and significant correlation was observed, indicating that as age increased, the severity of periodontal disease also increased (P < 0.001). On the other hand, no statistically significant relationship was found between the severity of periodontal disease and patients' gender (P = 0.709). Similarly, when comparing the relationship between the duration of diabetes and the severity of periodontal disease among the studied patients, no significant association was observed (P = 0.409).
5. Discussion
The proposed mechanism for the effect of diabetes on periodontal disease is the inflammation caused by diabetes, which significantly impacts periodontal tissue (16). Additionally, the increased severity of periodontal disease in diabetic patients may indicate changes in periodontal tissue that lead to faster deterioration. This progression can also be influenced by poor metabolic status (17-19). Chronic anaerobic periodontal infections may impair glycemic control and increase the risk of diabetes-related complications (20, 21). Poorly controlled diabetes is also considered a significant risk factor for the development and progression of periodontitis (22).
Epidemiological studies have shown that periodontal damage is significantly more common in diabetic patients, particularly those with type 2 diabetes (6, 23-25). Eldarrat demonstrated a significant relationship between blood sugar control and oral infections, as well as between the duration of diabetes and dental problems (26). Moreover, periodontal treatment has been shown to improve patients' metabolic control (27). However, some studies have reported no significant difference in the severity of periodontal disease between healthy individuals and diabetic patients (28, 29). Factors such as ethical and individual differences among patients in various studies, the severity of diabetes, and the medications used may influence these findings.
The present study was designed and conducted to assess the incidence of periodontal disease in diabetic patients. In most cases, the clinical course of periodontal disease is influenced by the systemic disorders present in patients. Cianciola et al. (30) reported a 39% prevalence of periodontitis in individuals over 19 years old, while Rylander et al. (25) reported an 87% prevalence in individuals over 35 years old. Additionally, Bacic et al. reported a prevalence rate of 50% (31).
The findings of the current study revealed a direct and significant relationship between glycemic control (HbA1c level), oral health status, and the severity of periodontitis. Consistent with previous studies, the prevalence of periodontal disease in this study was 88.25%, with all patients being over 35 years old. Similarly, Rajhans et al. reported a periodontal disease prevalence of 86.8% (32).
Sheridan (33) reported that the severity and prevalence of periodontal diseases increase with age. Similarly, studies by Albrecht et al. (34), Novaes et al. (35), and Bridges et al. (36) have compared periodontal status between patients with diabetes and non-diabetic individuals. Our findings also indicated that the severity of periodontal disease increased with age.
Collagen is one of the primary components of the connective tissue in the gums, comprising nearly 60% of the connective tissue volume and 90% of the organic matrix of the alveolar bone. Oliver and Oliver and Tervonen (37) highlighted that collagen content in the human body changes with age, a phenomenon that is more pronounced in diabetic patients with metabolic abnormalities. This suggests that alterations in collagen metabolism among diabetic patients contribute to the progression of periodontal disease in this population.
In our study, no significant relationship was found between the duration of diabetes and the severity of periodontal disease (P = 0.409). However, contrary to our findings, studies by Cerda et al. (38) and Firatli et al. (39) demonstrated a significant association between diabetes duration and periodontal disease severity. Similarly, Emrich et al. (40) reported that diabetes control status significantly correlates with both the prevalence and severity of periodontal disease.
Based on our study results, we concluded that patients with poor glycemic control (as indicated by HbA1c levels) experienced more severe periodontal disease. Karjalainen and Knuuttila (41) suggested that hyperglycemia in diabetic patients can lead to cell dysfunction, as glucose uptake in these patients requires insulin. Hyperglycemia can also impair the chemotaxis, phagocytosis, and intracellular destruction of bacteria by polymorphonuclear (PMN) cells. Additionally, prolonged hyperglycemia can lead to dysfunction in HbA1c, reducing tissue oxygenation. Furthermore, hyperglycemia can cause abnormalities in tissue blood flow, such as increased blood viscosity, reduced erythrocyte deformability, and increased platelet aggregation, all of which contribute to tissue hypoxia. Collectively, these factors may accelerate periodontal destruction in diabetic patients.
In the present study, 53% of patients demonstrated a good level of awareness regarding periodontal disease, while 64% had a positive attitude toward oral health. However, only 12% exhibited good oral hygiene practices, whereas 45% had poor performance in maintaining oral health. Periodontal diseases are among the common complications of diabetes, highlighting diabetes as a significant risk factor for the increased prevalence of periodontal conditions (42).
According to a review study conducted by Borgnakke et al. (43), available evidence suggests that periodontal diseases negatively impact diabetes outcomes. The authors emphasized the need for further longitudinal studies to explore this association in greater detail.
On the other hand, oral manifestations of diabetes are often observed in patients with poor oral hygiene (44), highlighting the importance of increasing awareness, attitude, and oral health practices among diabetic patients to improve their overall oral health status. Weinspach et al. also reported that enhancing patient awareness has been largely neglected in the dental treatment of diabetic patients (45). Additionally, a study by Noroozi et al. (46) found that diabetic patients had limited access to support resources for self-care, with personal adaptation to the disease serving as their most critical source of support.
Our findings regarding oral health status indicate that the majority of patients (72.25%) had an average oral health status, with toothpicks being the most commonly used oral hygiene tool (22%). Furthermore, 75.4% of patients required improved oral and dental hygiene, and only 11% used a toothbrush for oral care. In the study by Aggarwal and Panat (47), 22% of diabetic patients reported using a toothbrush twice a day, while in the study by Apoorva et al. (48), this percentage was 11%.
The present study has several limitations, including the single sampling center, the absence of a control group, the lack of comprehensive dental assessments, and its observational nature. Nonetheless, a review of our findings, along with recent studies, suggests that diabetes increases the risk of periodontal disease, particularly in cases of poor glycemic control. Available evidence also indicates that severe periodontal disease can contribute to disturbances in blood sugar regulation. Furthermore, treatment of periodontal disease has been associated with improved glycemic control, with recent meta-analyses reporting a 0.4% reduction in HbA1c levels following periodontal treatment.
5.1. Conclusions
Based on the results of this study, the incidence of periodontal disease among diabetic patients at Imam Khomeini Hospital in Urmia is 88.25%. Additionally, a direct and significant relationship was observed between glycemic control and the severity of periodontal disease in diabetic patients.