1. Context
Toxoplasmosis is one of the most common parasitic infections worldwide and almost one-third of the global population has carried the agent of this infection, namely Toxoplasma gondii (T. gondii) (1-3). However, the seroprevalence of this disease in the human population varies among nations from 9 to about 78% (4). Although armed forces are generally healthy, toxoplasmosis is not infrequent in this population. For instance, the seroprevalence of toxoplasmosis in military personnel in the Czech Republic was reported as 23% (4). Nevertheless, Colombian military personnel who were involved in jungle operations had 80% seroprevalence (5). Armed forces are expected to be exposed to transmission routes in battlefields and operation environments. Although toxoplasma infection is generally asymptomatic in immunocompetent individuals, there are some reports of epidemic outbreaks in military populations with fever, adenopathies and pulmonary and gastrointestinal compromise (6). Unfortunately, the chronic form of this disease may present neuropsychological manifestations (2). It has been suggested that retired armed forces and veterans, particularly those with chronic diseases, are susceptible to opportunistic toxoplasmosis. Therefore, medical services must investigate the potential of this disease and its complications in military populations. The aim of this review article was to describe changes in different parts of the brain during toxoplasmosis.
2. Evidence Acquisition
In this narrative review, we searched Google and Pubmed data banks as well as our department and the University library archives to retrieve studies during last 30 years about toxoplasmosis, brain and military forces. A total of 55 full papers were relevant and used in this review of which three were review articles.
3. Results
This parasite is an obligate intracellular protozoa that belongs to the Apicomplexa phylum without cilia or flagella and moves in the manner of gliding via its actin-myosin motor (7). It causes a zoonotic disease, where its definitive and intermediate hosts are cats and homeothermic animals for example humans, respectively (8-16). Infection initiates with an acute phase in healthy humans, which is mostly asymptomatic. However, it may convert to a chronic phase without symptoms; this phase being mostly localized in the brain (14). This is because T. gondii is able to pass the blood-brain-barrier and settle in the central nervous system (brain) and establish cysts including bradyzoites (17) that escape the immune system (3) and lead to chronic infection in the brain, and present various psychoneurological disorders (12, 18, 19).
3.1. Type of Parasite
In terms of polymorphisms of T. gondii genes, T. gondii includes three major strains (type):
Type Ι strain (RH) has been associated with acute virulence and congenital toxoplasmosis. This strain rapidly disseminates and reaches high tissue burdens because of its enhanced ability to cross biological barriers and penetrate deeper tissue layers such as lamina propria, submucosa, vascular endothelium and intestinal epithelium. It has notable capacity for transmigration and long distance migration (LDM). It also has been observed in ocular toxoplasmosis and AIDS victims. Type II strain is common in AIDS and immunosuppressive cases, and may lead to toxoplasma encephalitis. In ocular toxoplasmosis, type II strains are frequent and may be associated with schizophrenia. Type II also causes chronic infection with tissue cysts in mice, meanwhile it is the predominant strain that leads to infection among the population of Europe and North America and has also been recorded in patients with congenital disease. Type Ш strains are nonpathogenic (7, 20-31).
3.2. Host Gene
Host genes influence the outcome of clinical illness in toxoplasmosis besides other factors; for example, mouse is an excellent model for producing chronic toxoplasmosis but is a poor model for neurological features. The HLA-DQ genes in human and Ld gene in mice are important for regulation of immune responses against toxoplasmosis. Apparently, these genes control resistance or susceptibility of the host against toxoplasma encephalitis (21, 32).
3.3. Transmission
Human can be infected by T. gondii in different ways. A common route is the oral route and ingestion of various forms of parasite such as oocytes; including sporozoites which are found in soil, water, food, drinks and children's sandboxes contaminated with infected cat feces (1, 2, 7, 9, 12, 17, 33). Oocytes are ubiquitous in nature and are highly resistant to disinfectants and environmental influences and play a major role in fecal-oral transmission. Tissue cysts consisting of bradyzoites are found in raw or undercooked meat, and are associated with latent infections (1, 2, 33, 34). Finally, tachyzoites are transmitted as congenital infections via transplacental or by blood transfusion, organ transplantation and laboratory accidents (9, 24, 25, 27, 35). There is no reliable evidence for the prevalence rate of toxoplasmosis in animal populations. Cats are the definitive host of this parasite and can shed one million oocytes per gram of feces over a period of 1-3 weeks. Oocytes may survive in the outdoors for up to 18 months in soil and 24 months in seawater. This source, spreads infection to wild animals, domestic livestock and even coastal marine mammals (36). However, local studies have reported the transmission of toxoplasmosis to humans from various animal sources as intermediate hosts. In meat-producing animals, tissue cysts of toxoplasmosis are observed most frequently in sheep, goats, pigs and less frequently in rabbits (4). An old report demonstrated an outbreak of toxoplasmosis in army carrier pigeons in Panama in 1943 (37). Rodents and birds act as intermediate hosts and domestic animals and humans could be the end-stage of intermediate hosts.
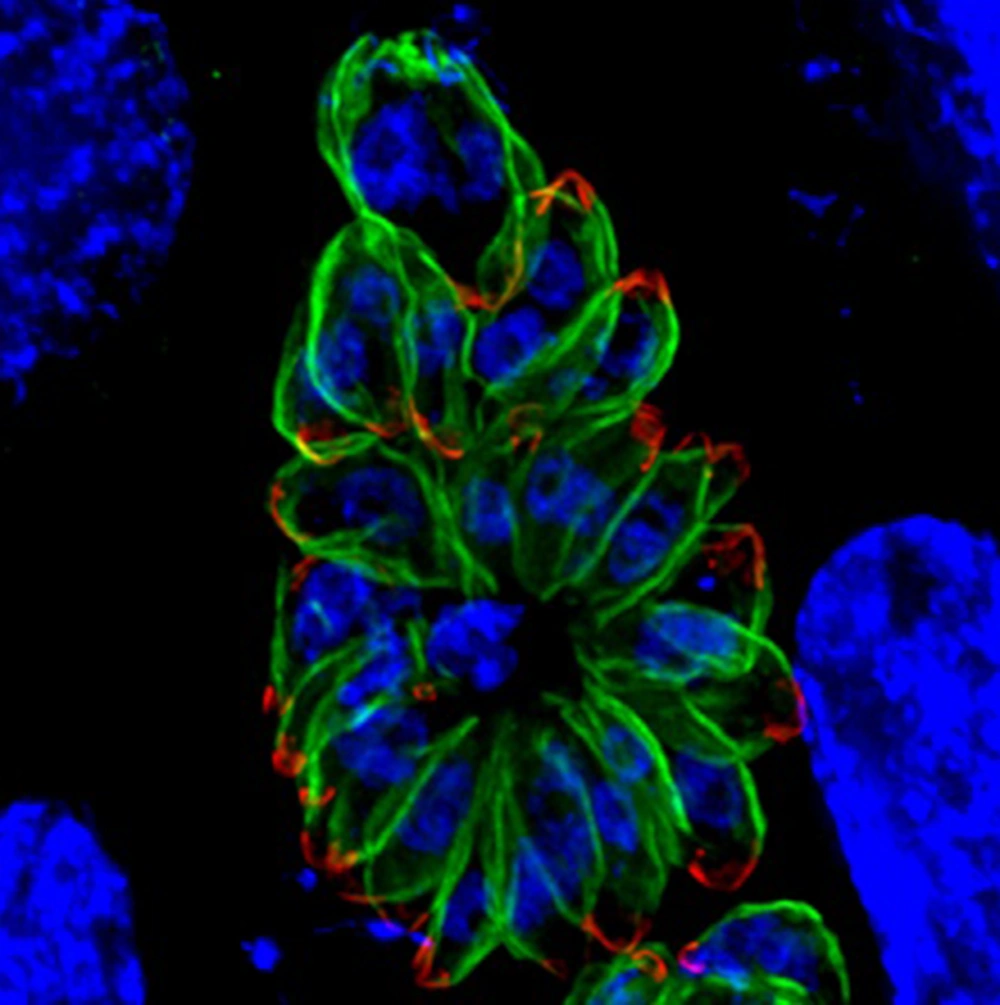
Note that in this mutant, parasite cells contain two nuclei. Nuclei are shown in blue, and the outline of the parasites are shown in green with a red apical cap (image credit for Maria Francia and Boris Striepen, University of Georgia, adopted with permission from PLoS Biology Issue Image | Vol. 10(12) December 2012. PLoS Biol 10 (12): ev10.i12. doi:10.1371/image.pbio.v10.i12).
Finally, mechanical vectors such as flies, cockroaches, dung beetles and earthworms must be considered as risk factors for toxoplasmosis transmission (36). Epidemiologic evidence have identified the following risk factors for toxoplasmosis transmission: owing cats, being in close proximity to seropositive cats in farming areas, cleaning cat litter boxes, eating raw or undercooked meat, gardening, soil contact, eating raw or unwashed vegetables or fruits, washing the kitchen knife infrequently, poor hand hygiene and traveling to endemic regions, in particular less developed countries with humid environments (34, 36). These conditions frequently occur during military deployment and field operations. Therefore, military forces could be considered as high risk for toxoplasma seropositivity. In a study on the Czech Republic Military Personnel, the overall seroprevalence was 23%, with the greatest rate in the age group of 30 to 34 years. In this study, the predictors of seroprevalence of toxoplasmosis included eating or tasting raw or undercooked meat, owing a cat, rabbit or dog and non-O blood groups (4). Also, contaminated water consumption is a potential source of transmission. Several outbreaks have been reported as a result of contaminated water sources in Canada, Brazil and India. The latter occurred after a rainfall in catchment areas infested with domestic and wild cats (6). A study on Colombian military jungle operating forces demonstrated 80% seroprevalence in comparison to 45% in soldiers in urban areas. The investigators suggested drinking un-boiled and chlorine untreated lake, stream or river water was a potential source of infection (5).
3.4. Life Cycle
T. gondii is an obligate intracellular coccidian protozoa. It causes a major zoonotic disease and cats are its definitive hosts. The sexual stage of the parasite life cycle occurs in cat intestines, producing immature oocytes within 1-3 days after contamination. Consequently oocyte release to the environment via feces and last 1-3 weeks. Oocyte maturation occurs in the environment during 1-5 days in proper conditions and sporozoites appear inside the cyst. The asexual stage occurs in the body of birds and all mammals as intermediate hosts, resulting in different forms of parasites including tachyzoites and bradyzoites (21-27, 29-31, 33). During the acute phase, the circulating tachyzoites in the blood invade different nuclear cells and establish in vacuoles to replicate rapidly in an endodyogeny manner (38). In the chronic phase, bradyzoites in tissue cysts are found in latent infections. T. gondii is an important protozoa that causes neuronal degenerative diseases, since tissue cysts, mostly affect the brain (35) and gradually lead to nerve degeneration. It is believed that bradyzoites, sporozoites and tachyzoites are different in gene expression, invasion, replication and migration but all forms are similar in possessing characteristic apical organelles; rhoptries, micronemes and dense granules that help them attach, penetrate the cell, and make parasitophorous vacuoles (11, 12). It seems that, the mode of the initial infection may strongly influence its duration and clinical manifestation (11).
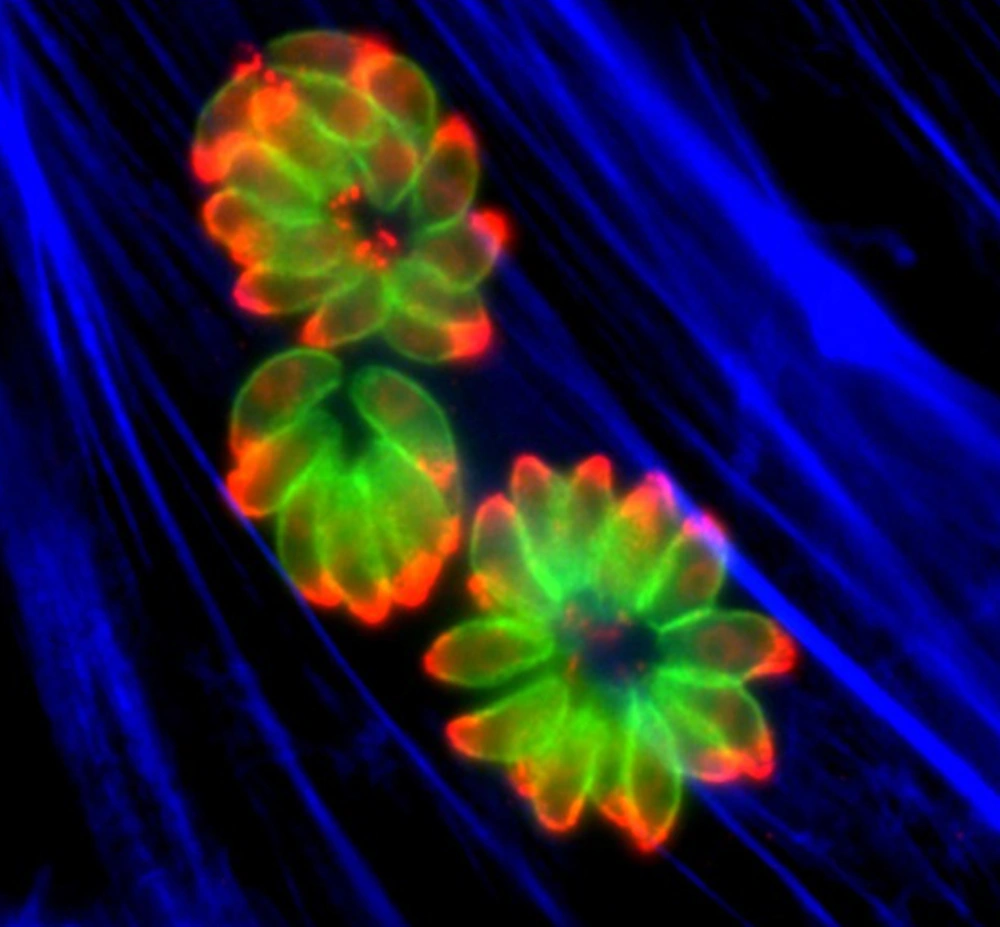
The progeny typically organizes as esthetic polarized rosettes before it exits from the vacuole and the host cell and disseminates throughout the host. To achieve this cycle, the tachyzoite has selected unique ways to reach and to invade host cells that by many aspects remain elusive (adopted from: L’Institut Cochin est un Centre de Recherche).
The parasite tries to pass any biological membrane such the intestinal or placental epithelium and blood-brain-barrier (BBB) in the form of free parasite (tachyzoite). The mechanism of passing through the BBB is poorly understood; however, a recent study suggested promotion of infected leukocyte migration through BBB in toxoplasma encephalitis (12, 39). In circulation, the parasite follows any nuclear cells such as macrophages and dendritic cells, and penetrates the cell membrane by different specific receptors on the cell surface (2, 25, 30, 31). Furthermore, the parasite then disseminates in the body as a “Trojan horse” (12) but it is unknown, whether it’s dissemination is in the form of free extracellular or intracellular parasite. The parasite doesn’t remain in the blood and attempts to run away from the immune system and enter into deep tissues (preferentially brain) and form tissue cysts. At this stage, the acute phase of infection converts to the chronic phase and creates a balance between the host and parasite (7, 38). In this chronic phase the parasite is predominantly found in the bradyzoite form within the CNS. Chronic settlement of bradyzoite cysts and the immunological response may result in behavioral modifications (12).
3.5. Immune Response
In general, the immune response against toxoplasmosis includes non-specific, specific and humoral immune responses. During non-specific immune responses, which continue for two weeks after infection, different cells such as macrophages, natural killer (NK) cells, antigen-presenting cells (APC), γ-δ T lymphocytes (TL), platelets, neutrophils, eosinophils, mast cells, fibroblasts and epithelial or endothelial cells are involved and lead to IL-12, IFN-γ and TNF-α secretion. Dendritic effector, CD8+ and CD4+ TL cells are involved in specific immune responses. CD4+ TL are divided into two sub-groups: Th1 (type-1) and Th2 (type-2). Type- 1 cells secrete IL- 2 and IFN-γ that along with IL-12 and CD8+ TL cells promote resistance and control the infection. On the other hand, TNF-α, TNF-β, IFN-γ and IL-2 have anti-inflammatory actions that make it possible to control the development of immune pathological phenomena related to a type-1 immune response (40). The Th2 cells produce IL-4, IL-5, IL-6 and IL-10. However, IFN-γ, IL-12, TNF-α, IL-6, IL-5, IL-15, IL-18 and IL-2 are the protective cytokines. IL-4, IL-10 and TGF-β (transforming growth factor-β) are regulatory cytokines. Furthermore, IgM and four sub-classes of IgG and IgA are the antibodies that appear in humoral response against toxoplasmosis and are useful in serological diagnosis. It has also been shown that appropriate humoral immune response promote the formation of intra-cerebral cysts (40). Infection leads to the secretion of a variety of cytokines by T-cells, blood derived macrophages, microglia, astrocytes and neurons, which affect inflammatory responses. Rupture of a tissue cyst is associated with a rapid influx of inflammatory cells and it is thought that the periodic rupture of these cysts lead to maintain immunity against T. gondii (40). The change from acute to chronic phases was associated with a change from a lymphocyte/monocyte population to one with a predominance of plasma cells. It is possible that strains elicit varying immunological responses, resulting in dissimilar alterations in neurotransmission as the three clonal lineages of T. gondii differ in their modulation of cytokine secretion. Mice deficient in T1/ST2 are more susceptible against the infection and increase the pathology and number of parasites in the brain. A number of hematopoietic cells such as T-cell, mast cells, macrophages, NK and invariant NK cells express the T1/ST2. Furthermore, ST2 also affects the down-regulation of the inflammatory response. IFN-γ has the most important role among cytokines secreted in response to T. gondii. IFN-γ dependent, cell-mediated immune responses and, to a lesser degree, by humoral immunity it suppresses the proliferation of tachyzoites during the acute phase of infection. This leads to latent toxoplasmosis and tissue cysts, primarily in the brain. In immunodeficient patients such as AIDS may reactive the infection by bradyzoites could be released following rupture of a cyst and invertes into tachyzoites. The lack of effective immune response and proliferation of tachyzoites lead to severe brain damage (8, 10, 14, 40, 41) and progression of toxoplasmic encephalitis. Animal models have demonstrated the interaction between the parasite and host immune response. T-cells are the main source of IFN-γ, which disseminate into the brain during the infection and play an important role in elimination of the reactivation of infection. T-cells with receptor Vβ8 are the major producer of IFN-γ in the brain of infected mice that are genetically resistant against toxoplasmic encephalitis. In addition, adoptive transfer of Vβ8 + T-cells alone, into infected nude mice (without T-cells) prevents the progression of toxoplasmic encephalitis. Thus, in murine models, these T-cells recognize the parasite antigens and are essential for the induction of protective T-cell response to prevent reactivation of infection. Apart from T-cells, other cells such as microglia and blood-derived macrophages produce IFN-γ in the brain of infected mice. The IL-33 receptor-signaling pathway plays a crucial role in the brain to control toxoplasmosis (42). It is essential to understand, how T. gondii effect brain cells; Blader et al. showed that within two hours after infection of fibroblasts with type ⅠⅠ strain of tachyzoite, expression of genes associated with chemokines (GRO1, GRO2, LIF, and MCP1) increased and led to the upregulation of cytokines (IL-1β and IL-6), then activation of transcription factors (REL-B, NF-κBp105, and I-κBα) and finally, promotion of immune response against the infection. Twenty-four hours later, tachyzoites increased up to 2-4 times. Next, it seemed that protein phosphatase 2C from rhoptries of tachyzoites played an important role to influence apoptosis and metabolic and antimicrobial mechanisms. The role of IL-8 in neutrophil activation may contribute to the early response to toxoplasmosis. Furthermore IL-6, has a protective role against toxoplasmosis and it has been observed that mice with deficiency of IL-6 are more susceptible to toxoplasmosis (40). It has been reported that astrocytes and microglial cells infected with T. gondii release IL-1α, IL-6 and granulocyte/macrophage colony-stimulating factor (GM-CSF). In addition, IL-6 may contribute to the exacerbation of autoimmune disorders in the CNS. Moreover, there is a significant association between IL- 6 and neurotransmitter production; other studies have reported that IL-6 may stimulate neurons to secrete dopamine and probably other catecholamines (40). Meanwhile, there are several mechanisms of parasite evasion, including parasitophorous vacuoles, apoptosis, renewal of antigens, and molecular mimesis, which share some epitopes with the host, such as cerebral epitopes. As a result the parasite remains undetectable in the brain and produces neurological complications such as schizophrenia (43).
3.6. Interaction Between the Host and Parasite
A complex interaction between the parasite and host is involved in toxoplasmosis, and produces some degree of harmony. To achieve latent, subclinical infection, a parasite-host homeostasis should be achieved. Different factors associated with both the parasite and the host such as the genetic background of the parasites and hosts and also the immune status of the hosts contribute to this balance (12, 44). On one hand, the immune cascade is necessary for this coexistence including many immune components and cells such as interleukins, cytokines, macrophage and other factors (21). On the other hand, the parasite should modify its way to survive and transmit, and avoid direct or indirect tissue damage by the immune response. Based on the immune response and the mechanisms of parasite evasion, the survival of the host cell and parasite are guaranteed (33, 45).
3.7. Anatomopathological Changes
Neurodegenerative disease patterns including significantly enlarged ventricles on MRI particularly near the aqueduct of sylvius (29) are frequently observed during para-clinical investigations. Dilatation of the ventricles of the brain of chronically infected mice revealed (22, 32), meningioma (46) while mild to moderate congestion (23) was also seen. Congenital toxoplasmosis can result in meningoencephalitis (47). Macroscopically normal volumes with patchy necrosis of the brain parenchyma have been noted in infected mice (20, 26, 42). Also, some reports demonstrated diffuse edema and congestion. However, in some studies no significant edema or necrosis was reported in parallel to the observed hemorrhage (19, 48). Diffuse meningoencephalitis, in the white and gray matter has also been seen (19).
3.8. Different regions of the Distribution of Cysts
Upon entry to CNS, tachyzoite parasites appear to infect astrocytes, neurons and microglial cells (12). Selective tropism of the parasite towards a particular functional system was not seen. Importantly the cysts were not preferentially related to the dopaminergic system and absent from the hypothalamic defensive system. A consistently low incidence of tissue cysts was recorded in the accessory olfactory bulb, cerebellum, pontine nuclei, caudate putamen and virtually the compact masses of myelinated axons. Among the five fundamental brain parts, the telencephalon showed the highest cyst density (20, 32). In contrary, the metencephalon was much less infected than expected from its volume. In the telencephalon a consistently high cyst density was seen in the external plexiform layer of the olfactory bulb, ventral pallidum and the entorhinal somatosensory motor and orbital cortices. Moreover, a high cyst density was seen in the frontal association and visual cortices, the tenia tecta and importantly the hippocampus and amygdala. Among non-telencephalic brain regions, the tegmentum, gigantocellular and medullary reticular nuclei showed a high cyst density in four out of five examined brains. Compact masses of myelinated axons for example the corpus callosum, anterior and posterior commissure, capsula interna, cerebellar peduncles, fimbria of the hippocampus and the hippocampal commissures featured a low incidence of cysts. On average the cyst density was 12 times higher in the gray matter than that of the white matter (20, 29). It is important to note that in the isocortex and the cerebellar cortex, cysts were most abundant within the molecular and/or external granular layers and within the molecular layer, respectively. The cysts were absent in the anterior hypothalamic nucleus, the dorsomedial part of the ventromedial nucleus and the dorsal pre-mammillary nuclei. The cysts were absent in the bed nucleus of the stria terminalis, infra-limbic cortex and lateral preoptic area. A high cyst density was seen in the anterior amygdaloid area, medial and cortical amygdaloid nuclei, while moderate cyst density was observed in the lateral, basolateral and basomedial amygdaloid nuclei and in the amygdalo hippocampal area. No tropism towards a particular hippocampal compartment was seen (20). Some studies did not report an increase in cyst density in the amygdalar structures (31). Cysts containing large numbers of bradyzoits were observed in all parts of the brain (23). Intriguingly, cysts showed a light preference for limbic system regions (9). The cysts seemed to be slightly more numerous in the hippocampus during acute parasite invasion and were commonly present in larger numbers in the amygdala during latent toxoplasmosis (49). Based on microscopic studies, cysts were present throughout the brain, but concentrated in the cerebral cortex, hippocampus, basal ganglia and amygdala (12). It has been reported that the density of cysts in the medial and basolateral amygdala is almost double that of other structures such as the hippocampus, olfactory bulbs and prefrontal cortex (12). The largest parasitic cysts and clusters were located at the core and shell of the nucleus accumbens and the ventromedial hypothalamic nucleus. Isolated toxoplasma cell and small clusters were also found in the frontal and cingulate cortex, medial preoptic area, amygdala, ventral tegmental area, central grey nuclei, raphe nuclei, and inside the ventricles and the aqueduct. The T. gondii was predominantly located in the medial portions of the brain i.e. in the prefrontal cortex, accumbens nuclei, hypothalamus and ventral tegmental area. Although the pattern of distribution was similar at different doses, the predominant location of parasites was in the limbic (predominant inversion) and medial areas such as the medical prefrontal cortex, cingulate hypothalamus and basal ganglia, in particular the nucleus accumbens. Parasite abundance was found in the amygdale (38).
3.9. Central Nervous System Cysts Characteristics
The size of the cysts ranged between 10 and 70 micrometers. They were found solitary or in groups of 2-10 in hematoxylin-eosin (H&E) prepared sections; on average about 80% of the cysts were solitary, 13% in pairs and 4% in triads. Large groups were rare. Average cyst size was 46.02 ± 5.08 micrometers (23) while 100 or more individual parasites were surrounded by a cyst wall produced during differentiation (12). Parasites were found scattered, isolated as clusters of varying sizes or as parasite cysts in the brain parenchyma in all infected animals as revealed by H&E staining (38).
3.10. The Probable Factors of Distribution
Various forms of toxoplasma infection distribution have been reported. These discrepancies may hypothetically be related to differences in host species and/or strain. The low level of infection in the cerebellum might be caused by cerebellar cytoarchitecture. The cerebellum features an extremely high cellular density, very small neuron and a few gelia to neurons ratio. Given that astrocytes are much more efficiently infected by T. gondii than neurons and that cerebellar granular neurons are still less efficiently infected in vitro than hippocampal neurons, it is tempting to assume that a high proportion of small tightly packed granular neurons constitute a limiting factor for T. gondii proliferation and subsequent cyst production. Finally, the white matter has a few cysts. This observation is in line with the fact that T. gondii encephalitis is in contrast to encephalitis of other etiology. Compact myelinated bodies may act as natural barriers for the parasite and cyto-architectural features such as the neuron size and glia to neuron ratio may influence the local parasite load. It has been suggested that a higher number of parasites may reach brain regions that are characterized by higher blood flow during the acute phase of infection. To support this “high blood flow-high parasite density hypothesis” (20, 38), a high number of parasite in olfactory bulb and primary visual, somatosensory and motor cortices that feature a high level of oxidative metabolism, and a rather low number of parasite in the piriform cortex and some association cortices that feature a moderate level of oxidative metabolism have been observed. The local blood flow generally relates to metabolic activity. However, accumulation of the parasite was reported in neither the retrosplenial and auditory cortices nor the subcortical regions though their main feature was a high metabolic activity. Thus, this hypothesis is not reasonable. Consequently, other factors e.g. local properties of the blood-brain barrier seem to be at work. All of the above factors can contribute interactively to increase infestation of certain cortical and subcortical regions (20).
3.11. Different Kinds of Brain Cells Involved in Infection of Toxoplasmosis
Cysts are formed in the neurons and glia (14, 29, 31, 41). In the neuron the cysts were present in all parts (dendrite, axon, soma) of the neuron (15). Tachyzoites invade microglia (12), and astrocytes and neuron bradyzoites develop within purkinje cells in the human cerebellum neurons and astrocytes (21, 42). There is evidence that astrocytes and microglial cells are infected by T. gondii. Astrocytes play a pivotal role in the production of kynurenic acid (KYNA) in the CNS, because astrocytes are the main source of KYNA. Likewise, astrocytes are one of the most important cells that are invaded by T. gondii. They degrade tryptophan by IDO (indoleamine 2, 3-dioxygenase) to inhibit T. gondii replication (22) and microglial and inflammatory nodules rapidly appear (10, 32). Accumulation of microglia was observed around the cysts. IFN-gamma as well as several other pro and anti-inflammatory cytokines and chemokines are generated by microglial cells after infection. Neurons are the dominant chronically infected cell types. Microglial cells may act as a "trojan horse" in the dissemination of recrudescent parasite infections. Immume-related pathology is also believed to be locally controlled by IT MP-1, as an inhibitor of matrix metalloproteinase (MMPS) produced by astrocytes and other microglical cells (12). In some cases, the T. gondii has been found inside the cell nuclei (38).
3.12. Rare Changes of Brain
It is very rare to encounter a brainstem granuloma due to T. gondii infection in such patients. Foci of spotty calcification were seen in addition to a few ruptured but calcified T. gondii cysts brain stem involvement with multiple cerebral lesions (27). Hydrocephalus is usually due to compression of CSF outflow pathway by ring enhancing lesions and can rarely be seen due to ventriculitis (50). Cerebellar toxoplasmosis in as HIV-infected with a poorly defined hypodense area located in both cerebellar hemispheres and vermis with apparent extension into the right middle cerebellar peduncle in an HIV-infected patient (8).
3.13. Chemical Changes in the Brain
Infection also induces the production of a variety of cytokines by microglia, astrocytes and neurons which activate or inhibit inflammatory responses (7). Deregulation of dopamine, production of pro-inflammatory cytokines, interferon-γ and indoleamine 2,3 dioxygenase change the levels, turnover and efficiency of many neuromodulators including dopamine, glutamate and serotonin. Furthermore, T. gondii may directly influence neurotransmitter levels (9, 12, 14, 20, 41). This is due to the inflammatory release of dopamine (DA) by increasing cytokines such as interleukin 2 (12). T. gondii directly increases local dopamine metabolism. More specifically T. gondii contains genes encoding a tyrosine hydroxylase, a rate-limiting enzyme of dopamine biosynthesis and the encysted parasite expresses dopamine in vivo and increases k+ induced release of dopamine from dopaminergic cells in vitro (20). This raises the possibility that T. gondii alters dopamine level by synthesizing its own tyrosine hydroxylase (9). Anxiolytic-like compounds change in dopamine transmission and decreases glutamate / GA13A ratio in the limbic area after astrocytes damage by up-regulation of inhibitory molecules (38). A study observed an increased level of DA but not serotonin or norepinephrine in the brain of mice with chronic latent infection at five weeks post-infection. In contrast, DA level was not found to be affected in a study on congenitally infected mice. Furthermore, DA signaling may be changed in the vicinity of T. gondii cysts. It is possible that T. gondii triggers the release of immune factors leading to downstream changes in glutamate N-methyl-D-aspartate (NMDA) and/or those effects on dopamine (29). There were some changes in brain concentrations of catecholamines and indoleamines in T. gondii infected mice. Mice with acute infections showed a 40% rise in homovanilic levels as compared with the controls. Dopamine levels however did not change. Norepinephrine levels in this group were 20% lower than that of the controls. The DA levels were 14% higher in mice with chronic infections than controls. Serotonin and SHIAA levels were not altered in infected mice (47). Concentrations of serotonin and S-HIAA did not change in the brain of animals during the infection (47). The 40% increase in Homovanillic acid (HVA) level in acutely infected mice might have led to the retardation in transport of HVA out of the brain. Norepinephrine levels were 28% lower in acutely (but not chronic) infected mice and DA was 14% higher in the chronic group compared to the controls. The significant decrease in norepinephrine (NE) in acutely infected animals suggests that activity of the enzyme, dopamine-beta-hydroxylase, may be reduced in noradrenergic neurons of infected animals. This enzyme is located in noradrenergic neurons but not in dopaminergic neurons, therefore the rate of conversion of DA to HVA in the latter may not directly affect NE formation in noradrenergic neurons. The primary rate-limiting step in NE as well as DA formation is at the level of the enzyme tyrosine hydroxylase; this enzyme catalyzes the first step in conversion of tyrosine to catecholamine. It is possible that the activity of this enzyme is reduced in noradrenergic neurons yet is usually active in the dopaminergic neurons of acutely infected animals. Dopaminergic neuron terminals are primarily located in the basal ganglia while those of noradrenergic neurons are primarily located in the brain stem (mid brain, pons and medula). The balance between synthesis and break down of catecholamine is upset (47) the alternation of nitric oxide levels in the brain resulting in the modulation of signaling pathways (31). Tryptophan is an essential amino acid for T. gondii replication and degradation of intracellular tryptophan by IDO, which is mediated by IFN-γ, inhibits intracellular replication of T. gandii (22). This organism affects levels of dopamine, norepinephrine and other neurotransmitters (25). Toxoplasmosis has shown to induce the expression of anti-apoptotic proteins: 13 cl2, Bf 11, BCL-x1, BCL-W, Mcl-1, Bad and Bas in host cells. The miRNAs are manipulated by T. gondii. The AKT pathway has been shown to be activated by this parasite. An interesting possibility is that mir-17-92 mediated decrease in levels of PTEN during T. gondii infection in brain cells could be activating AKT pathways, which may result in the development of brain cancers. Other miRNAS along with miR-17-92 can be affected by T. gondii (50). Levels of ST2 were shown to be upregulated in the brains of mice during T. gondii infection compared with uninfected controls. The increase of pro-inflammatory mediators is a direct consequence of a greater parasite burden. Increased IFNγ with a tissue specific astrocyte deletion of gp 130 is correlated with increased numbers of parasite in the brain (42). High testosterone individuals may be more susceptible to toxoplasmosis. In a small study, seronegative men were found to have lower concentration of testosterone than infected men (12). T. gondii increases dopaminergic activity or tryptophan metabolite levels. There is a 7-fold increase of KYNA in chronic T. gondii infected mice. Kynurenine hydroxylase is also stimulated by IFN-γ and in latent toxoplasmosis would accelerate the reduction of gray matter.
3.14. Histological Changes
Studies on mice demonstrated that necrotic lesions appear as lucent, vacuolated areas of variable size with pyknotic nuclei in H&E stained sections. Immunocytochemistry studies of the lesions showed a weak diffuse reaction with a few strongly stained toxoplasmas, predominantly towards the periphery of the lesions. Ultra-structurally, it was observed that the lesion consists of cell debris from general lysis of both neural and glial cells (32). In certain cases, numerous clumps of small crystals consistent with early calcification were observed among the cell debris. Only rare intact microglial or inflammatory cells were observed within the lesion. The few organisms present had the ultrastructural features of endozites. This type of lesion was also observed in great numbers (32). In addition small areas of dystrophic calcification were observed in only a few of the chronically infected mice (4 out of 32). One mouse was found to have a large zone of calcification. The incidence of dystrophic calcification was not affected by the period post-infection. There was no identifiable lesion on reaction to the areas of calcification. This rare dystrophic calcification observed in chronic infection develops from these lesions formed during the acute phase. During the chronic phase, the microglial/inflammatory nodules show some variations in structure and activity. The vast majority of cells consisted of macrophages filled with cell debris with no identifiable T. gondii antigens or parasites, admixed with a few lymphocytes and plasma cells. The presence of plasma cells was not observed in lesions during the acute phase. A few active lesions did contain antigens and/or parasites and these appeared to be related to a recent cyst rupture. These observations were consistent with the proposal by Frenkel and Escajadillo, suggesting that the nodules in chronic infections represent "tomb stones" to ruptured cysts. With an efficient immune response, neurological damage is limited to relatively small lesions, which are unlikely to result in any clinical symptoms. This would explain the benign nature of acquired toxoplasmosis infections. However, these lesions could have a cumulative effect leading to clinical symptoms. This could be consisted with the reported clinical symptoms in Panamanian night monkey and congenitally infected mice (32). It has been reported that there are progressive pathological changes during the first six weeks of infection. It was not possible to evaluate quantitative differences in the present experiments because of the different inoculums used to study the acute and chronic phase. The chronic phase (1-22 months) showed no difference with time; there was a continued presence of nodules and inflammatory changes that were reported for up to eight months (32). Furthermore, IL-13 (but not IL-4) minimized brain inflammation by inducing the death of activated microglia in the brain (42). Some studies reported multi micro-granulomatous encephalitis (13) and some studies represented "tomb stones", which are calcifications during chronic infection (32).
3.15. Clinical Consequences of Chronic Central Nervous System Toxoplasma Infection
T. gondii infected people are at a two-fold increased risk of brain cancers (46). The mechanism of this increased risk is not clear but inflammation may play a role. Meanwhile it has been shown that inflammatory responses may also promote schizophrenia and psychotic disorders. In term of IL-8, which plays a role in activation of neutrophils, its level increases during early toxoplasmosis, accompanied by IL-2 and IL-6 during schizophrenia. It has been reported that IL-2 and IFN-γ levels decrease during treatment with anti-psychotic drugs in schizophrenia patients (40). The dopamine imbalance between mesolimbic and mesocortical regions of the brain is suspected to contribute to the development of schizophrenia, delusion, auditory hallucination, obsessive-compulsive disorders, personality changes and risky behaviors (12). It may also result in impaired reaction time (51). Several epidemiological studies revealed an association between seropositivity for toxoplasmosis and vehicular accidents. In a retrospective study in Turkey, 33% of positive results was observed in accident victims compared to 8.6% positive rate in non-accident controls (12). Similarly, an increased odd of toxoplasma infection was reported in accident groups compared to non-accident controls by retrospective studies from Czech Republic. More recently, an increased incidence of traffic accidents in toxoplasma-infected military drivers has been reported by a large-scale prospective cohort study involving 3890 military drivers. In this study a high titer of toxoplasma antibodies in RhD negative individuals was accompanied with a six times higher rate of traffic accidents (51). From a different perspective, a link between toxoplasmosis and suicide has been reported (52). A study found that countries with high toxoplasma infection rates also had high suicide rates; however there are many potential confounders for this finding. A study on 45,000 women in Denmark showed that women with toxoplasma infection were 54% more likely to attempt suicide and were twice as likely to succeed (53). This may be primarily related to toxoplasmosis and neurosis and the impact of the immunologic response to toxoplasmosis on serotonine and dopamine levels (52). Thus, the increased rate of suicide among military deployed soldiers to endemic areas for toxoplasmosis such as the Middle East could be partially explained.
4. Conclusions
In summary, this review demonstrated that the telencephalon contains a higher cyst density (20, 32). On average the cyst density was 12 times higher in the gray matter than that of the white matter (20, 29). Parasite abundance was found in the amygdale and hippocampus (38). Some probable factors of cyst distribution in the brain are; 1- cytoarchitecture (cellular density), the ratio between neurons and glia; 2- compact myelinated bodies; 3- blood flow (20, 38); 4- oxidative metabolism (20). Glia cells are infected more than neurons and microglial cells may function as a "trojan horse" in the dissemination of recrudescent parasite infections (12). Some other changes like brain stem granuloma could also been detected (27). In terms of immunochemical response, the production of proinflammatory cytokines, IFN-γ and indoleamine 2-3 dioxygenase change the levels, turnover and efficiency of many neuromodulators including dopamine, glutamate and serotonin (9, 12, 14, 20, 41). T. gondii contains genes encoding tyrosine hydroxylase, a rate limiting enzyme of dopamine biosynthesis and expresses dopamine in vivo and increases K+ induced release of dopamine from dopaminergic cells in vivo (20). T. gondii increases dopaminergic activity or tryptophan metabolite levels (22). High testosterone individuals may be susceptible to toxoplasmosis (12). From a clinical perspective, acute toxoplasma infection is usually symptomless but may also present non-specific manifestations in particular in epidemics of military groups. Chronic infection may be accompanied by brain involvement and express neuropsychological presentations as well as an increased risk of neoplastic disorders. In conclusion, toxoplasmosis is frequent among military forces and CNS infection is likely in this population. Clinicians must be aware and investigate toxoplasma in suspicious neuropsychological manifestations even in immunocompetent and apparently healthy hosts, particularly for military deployed personnel to endemic areas.