1. Background
Burns happen during the wars, explosions and accidents and have high levels of pain among the injured soldiers. Healing phase presumably lasts too long that may lead to the hospitalization of the patient. Complications include physical, psychological and most importantly aesthetic concerns. Management comprises special strategies via specific treatment protocols; TSBS (total body surface area) index, which is expressed as a percentage, is used for triage and subsequent professional cares (1). A third-degree burn is the most disastrous and harmful deep injury that involves epidermis, dermis, nervous tissues and sudoriferous glands. There are particular treatment strategies to manage this problem: control of breathing rhythm, hemorrhage management, fracture closure, particle removal, infection control and control of body temperature (1, 2). Among different methods of burn classification, the traditional approach is a simple and applicable way, which categorizes the injury into first, second and third degrees. In a third degree burns, in addition to epidermis and dermis, subcutaneous tissue and muscles are also involved. Tissue repair then initiates via healing process including three distinct phases: 1) inflammatory or acute phase, 2) granulation phase and 3) scar remodeling or healing phase (3). Recently, the bulk of scientific studies indicates that laser photostimulation could enhance wound healing process under in vivo and in vitro conditions; some of these researches suggested possible mechanisms of wound healing acceleration such as: collagen synthesis (4), fibroblast and chondroblast proliferation (5, 6), reducing osteoclastic activity and increasing of blood flow (7), nerve regeneration (8), stimulation of keratinocyte proliferation (9) and anti-inflammatory effects (10). The major characteristics of low intensity lasers are: power (range between 1-500 mW), mode (continuous or pulsed), dose (J/Cm2), exposure time and wavelength. It has been suggested that the low level laser therapy in the range of green light wavelengths (530 nm) is beneficial for the treatment of pigmented lesions and tattoos (3, 11). However, a few articles indicated the role of blue light wavelengths (400 nm) in treatment of acne and other inflammatory skin lesions (12-16).
2. Objectives
Regarding the proposed influences of laser photostimulation on wound healing process, the aim of this study was to examine the effect of the two different wavelengths of Ga-As laser (405 nm and 532 nm) on healing of third-degree burn injuries from both a dermatological and histological standpoint of view.
3. Materials and Methods
3.1. Animal Study
This study was conducted on 36 male Wistar rats (8-week-old, weighting 250-300 g) obtained from Pasteur Institute of Iran. During the study, all rats were kept in the separated cages under standard environmental condition (12-h light/dark cycle, temperature ~ 23˚C) and provided with standard laboratory food and ad libitum water. Animal protocols were under the supervision of the Institutional Animal Care and Use Committee of AJA University of Medical Sciences. Animals were anesthetized with intraperitoneal injections of ketamine and xylazine (30 mg/kg and 10 mg/kg body weight, respectively) . Dorsal body hairs were shaved and third-degree skin burns were created on the back of the animals (neck area) by using a copper stamp (length: 2.2 cm, width: 2 cm and height: 5 mm). The stamp was warmed up first to 95˚C via a heater with a digital controller and after each attempt it was held on the heater for one minute for making the next third-degree burn. Before doing so, in a pilot study, the stamp was used on a non-experimental rat to test if a third-degree skin burn could be made. After burning, the injured skin was immediately incised and it was observed that the fascia and the muscle were involved. Afterwards, the animals were randomly divided into three subgroups:
1) Green laser group (G) that was irradiated by 532 nm wavelength
2) Blue laser group (B) that was irradiated by 405 nm wavelength
3) Control group (C) that left untreated
Length and width of each wound were measured by a caliper and the area of the lesion was calculated on first, third, seventh, tenth, fourteenth, seventeenth, and twenty-first days of the study. All animals were weighed on the same days. On the last day of the study, six samples were taken from each group for histological analysis.
3.2. Laser Therapy
Lesions of green and blue laser groups were irradiated by use of Ga-As laser (LASER SYSTEM Ltd. Iran) with predetermined wavelengths (Figure 1). The characteristics of laser devices were:
1) Wavelength: a) green: 532 nm, b) blue: 405 nm
2) Power output: 20 mW
3) Spot size: 1 cm2
4) Exposure time: 75 seconds (this number is calculated according to power, spot size, exposure time and treatment dose)
5) Mode: continuous
6) Application mode: non-contact at the distance of 2 mm from the skin lesion
7) Dose: 1.5 J/cm2
3.3. Dermatology
In the present study, the only quantitative variable for dermatologic analysis was “wound contraction” on the third, seventh, tenth, fourteenth, seventeenth and twenty-first days. As mentioned earlier, after measurement of length and width of each lesion, size diminution of the wound area considered as the primary indicator for healing promotion assessment.
3.4. Histology
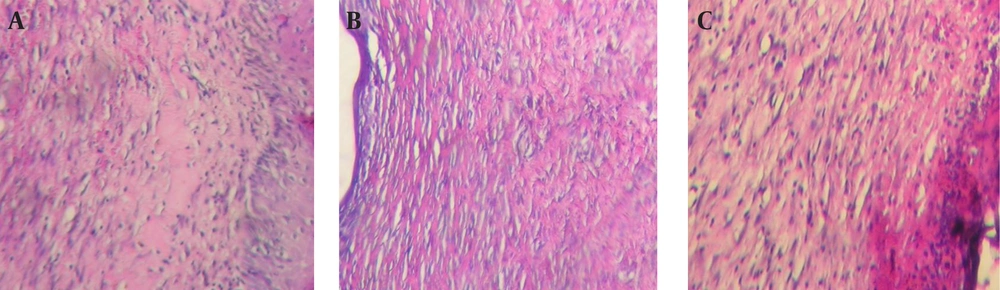
On the twenty-first day of the study, six lesions in each of three study groups were excised with safe margins and were fixed in 10% buffered formaldehyde for 24 hours. The samples were cut longitudinally (4 μm thickness) with microtome and were embedded in paraffin. Later on, they colored by the routine H&E staining protocol. Glass slides were prepared and evaluated by a pathologist who was not aware of the sample cods. By using a light microscope (Nikon E400, Japan), the following histological parameters were analyzed: epithelial continuity, existence of skin accessory structures, type of connective tissue (fibrous or granulation tissue), and type (acute or chronic). The photographs then obtained with a digital camera (Nikon E8400, Japan).
3.5. Statistical Analysis
As data collected from calculating the wound contraction area and body weight of animals according to study design, statistics were used as a main tool to explain the concepts and interrelations of the variables. The results were calculated using Prism software (version 5.0 for windows 1992-2007 GraphPad Software, Inc). Statistical methods for interpreting data in this study were:
1) Descriptive statistics (mean, standard deviation) were calculated for all variables and tabulated afterwards.
2) Kruskal-Wallis and two-way ANOVA tests were used to calculate the mean differences of wound contraction in millimeter among all groups on all study days.
3) The Non-parametric Friedman test was used to calculate the mean differences of wound contractions in millimeter within all groups of the study.
4. Results
4.1. Clinical Findings
4.1.1. Between Groups
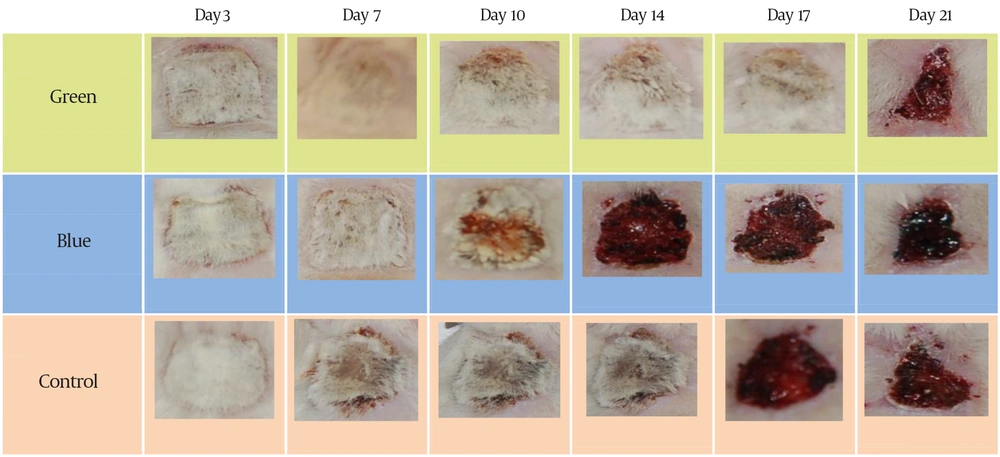
Statistical analyses of the “wound contraction” changes between three groups during the study are shown in Table 1. No significant statistical difference was found between the study groups. For the first and third days, we used Kruskal-Wallis test because measurement variables were not normally distributed. For other days, the ANOVA test was used for calculating mean differences of the measurements. Figure 2 shows the sequence of healing in three samples (B, G, and C) from the first until the last study days.
| Day | Group | No. | Mean or Mean-Median | Standard Deviation | P Value |
|---|---|---|---|---|---|
| 1 | G | 4.4-4.4 | 0 | 1 | |
| B | 4.4-4.4 | 0 | 1 | ||
| C | 4.4-4.4 | 0 | 1 | ||
| 3 | G | 4.33-4.4 | 0.13 | 0.407 | |
| B | 4.36-4.4 | 0.11 | 0.407 | ||
| C | 4.38-4.4 | 0.05 | 0.407 | ||
| 7 | G | 12 | 3.9300 | 0.44095 | 0.25 |
| B | 12 | 3.8525 | 0.46833 | 0.25 | |
| C | 12 | 4.1267 | 0.28823 | 0.25 | |
| Total | 60 | 3.9927 | 0.36419 | 0.25 | |
| 10 | G | 12 | 3.6508 | 0.53771 | 0.523 |
| B | 12 | 3.4800 | 0.56665 | 0.523 | |
| C | 12 | 3.6992 | 0.33544 | 0.523 | |
| Total | 60 | 3.5710 | 0.49615 | 0.523 | |
| 14 | G | 12 | 3.3342 | 0.63200 | 0.643 |
| B | 12 | 3.1325 | 0.68998 | 0.643 | |
| C | 12 | 3.3283 | 0.42910 | 0.643 | |
| Total | 60 | 3.2097 | 0.61412 | 0.643 | |
| 17 | G | 12 | 1.9913 | 0.78746 | 0.748 |
| B | 12 | 2.0692 | 0.93262 | 0.748 | |
| C | 12 | 2.2288 | 0.55886 | 0.748 | |
| Total | 60 | 1.9859 | 0.67508 | 0.748 | |
| 21 | G | 12 | 1.1863 | 0.65754 | 0.174 |
| B | 12 | 1.7600 | 0.96580 | 0.174 | |
| C | 12 | 1.6667 | 0.69495 | 0.174 | |
| Total | 60 | 1.1863 | 0.70252 | 0.174 |
4.1.2. Within Groups
Statistical analyses of the “wound contraction” changes within groups of the study during the research are shown in Table 2-4. For all existing groups in all days of the study, the result of the Friedman test revealed the statistically significant differences in wound size reduction in the green andblue laser and control groups (P < 0.05).
| No. | Mean | Standard Deviation | 50th (Median) | P Value | |
|---|---|---|---|---|---|
| DAY1 | 12 | 4.4000 | 0.00000 | 4.4000 | |
| DAY3 | 12 | 4.3325 | 0.13261 | 4.4000 | |
| DAY7 | 12 | 3.9300 | 0.44095 | 3.9600 | 0.00 |
| DAY10 | 12 | 3.6508 | 0.53771 | 3.6050 | |
| DAY14 | 12 | 3.3342 | 0.63200 | 3.4200 | |
| DAY17 | 12 | 1.9913 | 0.78746 | 2.1700 | |
| DAY21 | 12 | 1.1863 | 0.65754 | 0.9800 |
| No. | Mean | Standard Deviation | 50th (Median) | P Value | |
|---|---|---|---|---|---|
| DAY1 | 12 | 4.4000 | 0.00000 | 4.4000 | |
| DAY3 | 12 | 4.3667 | 0.11547 | 4.4000 | |
| DAY7 | 12 | 3.8525 | 0.46833 | 3.9950 | |
| DAY10 | 12 | 3.4800 | 0.56665 | 3.5000 | 0.00 |
| DAY14 | 12 | 3.1325 | 0.68998 | 3.1450 | |
| DAY17 | 12 | 2.0692 | 0.93262 | 1.8900 | |
| DAY21 | 12 | 1.7600 | 0.96580 | 1.3200 |
| No. | Mean | Standard Deviation | 50th (Median) | P Value | |
|---|---|---|---|---|---|
| DAY1 | 12 | 4.4000 | 0.00000 | 4.4000 | |
| DAY3 | 12 | 4.3833 | 0.05774 | 4.4000 | |
| DAY7 | 12 | 4.1267 | 0.28823 | 4.2000 | |
| DAY10 | 12 | 3.6992 | 0.33544 | 3.6900 | 0.00 |
| DAY14 | 12 | 3.3283 | 0.42910 | 3.4000 | |
| DAY17 | 12 | 2.2288 | 0.55886 | 2.3150 | |
| DAY21 | 12 | 1.6667 | 0.69495 | 1.4500 |
4.2. Histology
Photomicrographic samples of all three groups showed similar results at the end of twenty first day of the study. In almost all samples, some areas of epithelial discontinuation were observed clearly. However, inflammation was still evident even after twenty one days in most samples and existence of both fibrous and granulation or mixed tissues were observed in all groups. Accessory skin structures were not completely formed in most of the samples and dominant type of inflammation was chronic (Figure 3).
5. Discussion
We conducted our study on rats to investigate the effect of LLLT on the healing process via evaluating “wound contraction” and histological outcomes. Our observation included more rapid “wound contraction” in all laser groups in all study days (3rd, 7th, 10th, 14th, 17th and 21st days) as compared with the control group. For all study groups, the amount of wound contraction on all measuring days was statistically significant in comparison with previous day. For the green laser group, the greatest contraction was observed after fourteenth day that might show its effective action in granulation and scar-remodeling phases of healing process. The mean of wounded area for the blue laser group was changed with an almost constant work on from the third until seventeenth day of the investigation. However, after seventeenth day, the healing process become slowly and on the last day, the wound contraction was even less than the control group, which might reveal its inhibitory effect of this wavelength in this stage of healing process. For the control group, healing process showed a more constant pattern in all three stages and changed from 4.4 mm2 on the first day to 1.66 mm2 on the last day of the study. On the third day of the study, the amount of wound closure was almost the same between all the study groups; however, samples of the green laser group somehow revealed more wound contraction. On the seventh day, the pattern was changed and became restively constant until the fourteenth day. In this portion of time, both laser groups showed a significant difference compared with the control group with more wound contraction for the blue laser group. On the seventeenth day, we observed a greater wound contraction in samples that were radiated with the green laser, however, the pattern of wound size reduction in the blue and control groups was becoming more similar gradually. This similarity took its peak on the last day of investigation. Furthermore, reduction of wound diameter in blue group from the beginning of the study until day 14 was greater than the green group, which may due to its specific anti-inflammatory role as mentioned by some articles. Overall, our findings were in contrast to the findings of those investigators who found out the significant effect of LLLT on accelerating repair process of injured tissues during animal lab experimental studies ,which might be due to different methods specially dissimilar dosage and the rhythm of exposure (4-10). In contrast, our findings were in accordance with those studies who did not find any statistical significant positive effect of soft laser on wound healing process (17-19). By investigating the role of blue laser on inflammatory cytokines, Shnitkind and coworkers stated that narrow-band blue light had anti-inflammatory effects on keratinocytes through decreasing the cytokine-induced production of IL-1 alpha and ICAM-1 (16). Ammad and coworkers studied the effect of blue laser (415-425 nm) on treatment of acne vulgaris and concluded that it may have some beneficial influence on mild and moderate types of these lesions (12). After day 14, the pattern was changed and the green group showed more “wound contraction” in comparison with the blue group which may reveal its accelerating role on wound repair during granulation and healing phases. However, this difference was not statistically significant in acute, granulation and healing phases. Histological findings failed to show any remarkable advantage of laser groups over the control group. Nevertheless, statistical results of our study showed that using low level laser therapy with green and blue wavelengths may accelerate healing process as compared to the control group; however, this effect was neither statistically nor histologically significant. From this study, it is still unclear that the lasers with aforementioned wavelengths could clinically help the soldiers to be healed more quickly with less pain experience. Therefore, more specific researches must be performed to find out the advantages of this kind of healing method on burn injuries. Our results did not confirm any significant positive influence of LLLT with given protocol on tissue healing process in third-degree burn injuries in rats. However, a mild anti-inflammatory effect of blue laser was observed during the acute phase. Further studies should be performed to investigate other protocols and prescriptions of LLLT to clarify its role in the treatment of wounds and burn injuries.