1. Background
Inappropriate use of antibiotics is one the most important challenges for health systems. Irrational antibiotic consumption could result in increasing antibiotic resistance (1). It causes health professionals to use alternatives, which are more toxic and expensive. Moreover, antibiotic resistant bacteria are associated with higher morbidity and mortality of patients and subsequently increase the cost of health care (2). Therefore, health system policy makers have been tried to implement reasonable administration guidelines for antibiotics through appropriate drug policies (3).
Bacterial resistance to antibiotics is rapidly increasing in the world due to inappropriate use of antibiotics (4). Unnecessary prescription of antibiotics, improper selection of antibiotics and wrong dose prescription have important roles in bacterial resistance. At least, one of three patients admit to hospital receives antibiotic therapy, while it is unnecessary for a half of them (5). Previous studies showed that 30% - 50 % of prescribed drugs are antibiotics, while it was inappropriate in 30% - 60% of antibiotic prescriptions. This inappropriate use of antibiotics has resulted in spreading antibiotic resistance strains of Staphylococcus aurous, Pneumococcus, Enterococcus and Gram-negative intestinal bacteria in the recent decades (6, 7). Antibiotics use was about 12% of the total drug use in 1990, while it increased to 34% in 2000 and its cost was about 40 billion US Dollars in the world. Despite efforts to decrease antibiotic use, reports showed its increase worldwide (8).
Based on previous studies in two recent decades in Iran, antibiotics were the best-seller among all other drugs (9). These studies often considered overall antibiotic consumption in Iran or outpatient antibiotic use only. Antibiotic use pattern in Iran hospitals has not been adequately addressed.
In the hospital setting, intensive care unit (ICU) is a unique place in generating antibiotic-resistant pathogens and transmission of them between patients. It is due to use of broad spectrum antibiotics and impaired host defense (10). Antibiotic resistance in ICU is a worldwide challenge, especially in low and middle income countries (11).
2. Objectives
As excessive and irrational use of antibiotic drugs is one of the main reasons increasing the resistance of pathogens and there are limited evidences in Iran, in this study, we aimed to evaluate antibiotics use pattern in ICUs of five hospitals in Tehran, Iran.
3. Materials and Methods
3.1. Hospital Settings and Data Collection
This was an observational cross-sectional research. The study included five military hospitals in Tehran, Iran. Four of them were general hospitals with various medical and surgical wards while one of them (hospital B) was a heart center. General characteristics of hospitals obtained from hospital annual report for deputy of clinical affairs of related university. Average active beds varied from 59 to 459 beds. See details about the hospitals and ICU wards in Table 1.
| Hospital | Active Beds and Wards | Active Beds | Occupancy, % |
|---|---|---|---|
| A | 154 | ||
| ICU | 10 | 97 | |
| B | 59 | ||
| ICU | 7 | 38 | |
| C | 459 | ||
| ICU 1 | 8 | 99 | |
| ICU 2 | 8 | 97 | |
| ICU OH | 8 | 60 | |
| D | 194 | ||
| ICU | 7 | 95 | |
| E | 134 | ||
| ICU 1 | 6 | 89 | |
| ICU2 | 9 | 86 |
Data related to antibiotics use in ICUs were collected from 20 March 2011 to 20 March 2012 through hospital’s health information system (HIS) retrospectively at month level. The collected data included all drugs requested by the ward and provided by hospital pharmacy. So we separated antibiotics from collected data.
Some drugs ordered by physicians may not be provided by hospital pharmacy and patients obtained them from other central pharmacies in Tehran. Therefore, we evaluated one day antibiotic use of the ward to determine missed antibiotics. Then we adjusted our collected data.
3.2. Construction of the Indicator
In this “drug use evaluation” study, we used the anatomical therapeutic chemical (ATC) classification system and the defined daily dose (DDD) introduced as a gold standard measuring unit for international, national and local drug use research by the WHO collaborating centre for drug statistics methodology (12). We used ATC codes to determine antibiotics (ATC-group J0; Antibacterial for systemic use).
We converted antibiotic usage data to DDD. “The DDD is the assumed average maintenance dose per day for a drug used for its main indication in adults”. DDD would be assigned only for drugs with ATC code (12, 13). Then we expressed data as Defined Daily Dose per 100 Bed Days (DDD/100 Bed Days) based on the below formula (Equation 1) (14, 15);

However, drug use 90% (DU 90%) was determined for all ICUs antibiotic consumption. Drug use 90% includes all antibiotics used as 90% of all antibiotic consumption.
3.3. Analysis
Data analyzed by SPSS software version 16 (Chicago, SPSS Inc.) using one way ANOVA and Tukey post hoc tests to compare antibiotics use in ICUs between different hospitals. P value less than 0.05 was considered as statistically significant.
4. Results
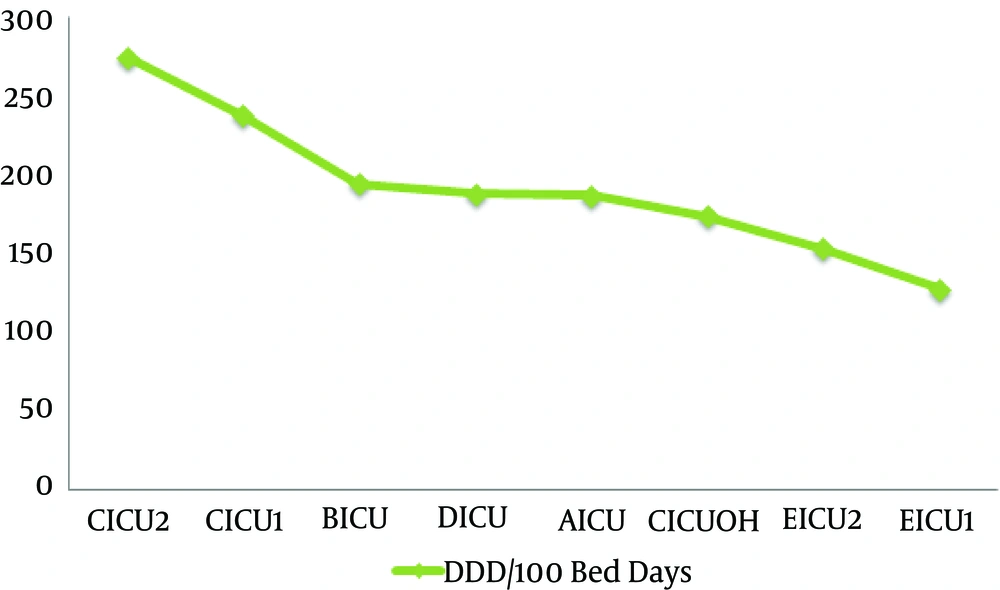
Antibiotics use in ICUs assessed monthly and annually based on DDD/100bed days. Findings showed that ICU2 and ICU1 wards in hospital C had the most annual use of antibiotics, respectively (274.4 and 238.04 DDD/100Bed Days).
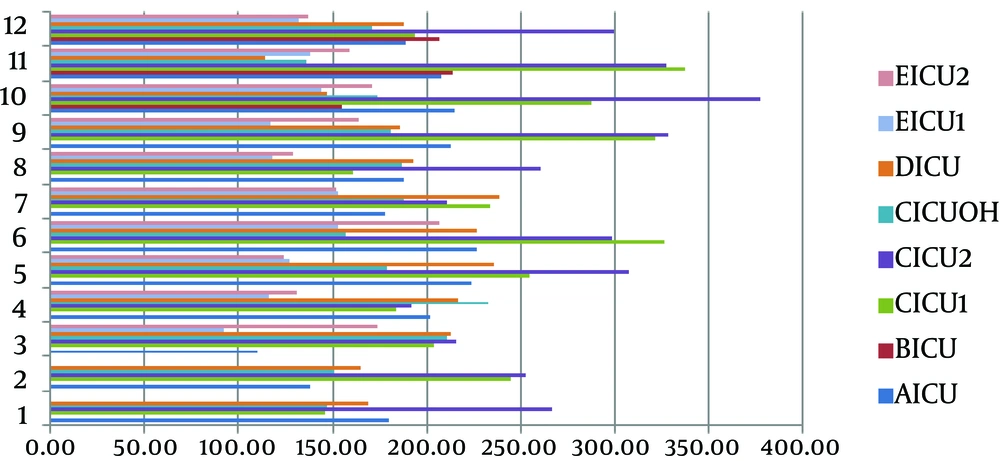
Analysis of Variance and Post Hoc Tests showed that antibiotics use in ICU2 ward of hospital C is significantly higher than hospitals A, D, E and ICU-Open Heart (ICUOH) of hospital C (P value ≤ 0.001). Use of antibiotics in ICU1 ward of hospital C was also higher significantly compared to hospitals D, E and ICUOH of hospital C (P value ≤ 0.001). Figure 1 shows a histogram for annual antibiotics use in ICUs. Figure 2 shows a clustered bar chart of monthly antibiotic use in ICUs.
Drug use 90% profile of hospitals’ ICU wards showed that different antibiotics had the most consumption. Beta-lactams, Cephalosporin and Vancomycin were the most antibiotics used in these wards. See details in Table 2.
| Hospital A | Hospital B | Hospital C | HospitalD | Hospital E | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibiotic | DDD | Percent | Antibiotic | DDD | Percent | Antibiotic | DDD | Percent | Antibiotic | DDD | % | Antibiotic | DDD | Percent | |
| 1 | Vancomycin | 1,082.00 | 16.30 | Ceftazidim | 212.75 | 37.10 | Ampibactam | 3,144.00 | 17.70 | Ceftriaxone | 1,100.50 | 23.90 | Meropenem | 1009.5 | 18 |
| 2 | Ceftriaxone | 1,078.50 | 16.30 | Amikacin | 162.50 | 28.30 | Meropenem | 2,052.00 | 11.50 | Meropenem | 707.50 | 15.40 | Ceftriaxone | 863.5 | 15.4 |
| 3 | Meropenem | 780.00 | 11.80 | Vancomycin | 131.25 | 22.90 | Ceftriaxone | 1,967.75 | 11.10 | Vancomycin | 664.00 | 14.40 | Vancomycin | 834.5 | 14.9 |
| 4 | Ciprofloxacin | 749.50 | 11.30 | Cefazolin | 1,892.20 | 10.60 | Cefepime | 380.00 | 8.20 | Ciprofloxacin | 635.5 | 11.3 | |||
| 5 | Cefazolin | 611.67 | 11.30 | Vancomycin | 1,438.50 | 8.10 | Metronidazole | 355.67 | 7.70 | Imipenem | 558.75 | 10 | |||
| 6 | Metronidazole | 448.05 | 6.70 | Clindamycin | 1,215.33 | 6.80 | Clindamycin | 255.00 | 5.50 | Tazocin | 252.57 | 4.5 | |||
| 7 | Imipenem | 438.25 | 6.60 | Amikacin | 1,140.50 | 6.40 | Cefazolin | 207.00 | 4.50 | Clindamycin | 232 | 4.1 | |||
| 8 | Clindamycin | 314.25 | 4.70 | Ciprofloxacin | 926.05 | 5.20 | Imipenem | 197.25 | 4.30 | Azithromycin | 206.67 | 3.7 | |||
| 9 | Gentamycin | 227.52 | 3.40 | Tazocin | 836.00 | 4.70 | Tazocin | 135.86 | 2.90 | Ampibactam | 177 | 3.2 | |||
| 10 | Ceftazidim | 183.25 | 2.70 | Metronidazole | 615.33 | 3.40 | Azithromycin | 116.67 | 2.50 | Ceftazidim | 169.75 | 3 | |||
| 11 | Azithromycin | 492.50 | 2.80 | Cefazolin | 128.33 | 2.3 | |||||||||
| 12 | Imipenem | 313.50 | 1.70 | ||||||||||||
| 13 | DU90% 1 - 10 | 89 | DU90% 1 - 3 | 88.3 | DU90% 1 - 12 | 90 | DU90% 1 - 10 | 89.3 | DU90% 1 - 11 | 90.4 | |||||
5. Discussion
In this study, annual antibiotic use in ICUs of five hospitals in Tehran was assessed. Findings showed that ICU ward of hospital C had the highest consumption and hospital E the lowest consumption of antibiotic annually. Determinants could influence it including different physicians, ward or hospital management or different case mix. Antibiotic use pattern was different among studied ICU wards while Cephalosporin, B-lactams and Vancomycin were the most used antibiotics.
Comparison of studied ICUs with some European countries showed that annual antibiotic use is higher in most Tehran hospitals than European ones (12, 14).
The results of a study in the hospital’s intensive care unit in Qazvin showed that pattern of antibiotics usage, high use rate and irrational prescription in intensive care unit, inadequate hospital and national drug policies, loss of antibiotic multidisciplinary team and inadequate infectious specialist consultation are very important issues in reduction of antibiotics use (16).
For most used antibiotics, the pattern is different in other countries’ hospitals from our studied wards. Previous studies in Europe and southern and eastern Mediterranean hospitals showed that in almost all of the hospitals, penicillin was the main antibiotic consumed in hospitals and then Cephalosporin used more than other antibiotics (17-22). Overuse of expensive antibiotics such as Vancomycin and Carbapenems in our studied wards resulted in extra cost for patients, hospital and health system. Different factors may be attributed to the antibiotic use pattern in our studied ward. Lack of proper drug use policies could be resulted in lack of appropriate protocols, guidelines and formulary books. These factors affect antibiotic use patterns in hospitals. Inappropriate monitoring and evaluation of antibiotic use and microbial resistance, lack of continued medical education and lack of pharmacologist or clinical pharmacologist are the other associated factors which may cause over-and misuse of antibiotics in hospitals (23, 24). The results of a study in Ahvaz hospital showed that about 42% of patients received appropriate prophylactic antibiotics and the most common mistakes were antibiotic selection followed by prolonged prophylaxis (> 24 hours) and excess dosage (25). The results of a study in India showed that antibiotics are commonly prescribed to most ICU patients at admission and contribute significantly to the total drug costs. Antibiotic restriction policies and a multidisciplinary effort to reduce usage are urgently required (1).
In all studied hospitals, there was no regular monitoring system. Therefore, one of the limitations of this study was obtaining data from hospitals regarding antibiotic use in different years and comparing them together. It was very time consuming to separate antibiotic use data in different wards due to lack of a strong health information system.
This study showed that the use of antibiotics was higher than expected in the studied ICUs, but antibiotics use pattern was different from other countries. Therefore, this problem should be addressed to correct the use pattern of antibiotics in ICUs.
Some suggestions including implementation of antibiotic use and microbial resistance monitoring programs, continuous medical education for physicians and compilation of clinical practice guidelines and protocols could be effective in reduction of antibiotic use in hospitals. Also performing other similar studies in other provinces of our country as well as trend analysis for an assessment during time can be proposed.